More Information
Submitted: December 12, 2022 | Approved: December 16, 2022 | Published: December 20, 2022
How to cite this article: Paerhati P, Xia PH, Tao NL, Hua GY, Fang LC, et al. Optimization of the fermentation process, characterization and antioxidant activity of exopolysaccharides produced from Azotobacter As101. Ann Adv Chem. 2022; 6: 082-088.
DOI: 10.29328/journal.aac.1001036
Copyright License: © 2022 Paerhati P, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Optimization of the fermentation process, characterization and antioxidant activity of exopolysaccharides produced from Azotobacter As101
Paiziliya Paerhati1,2, Ning Hui Xia1, Niu Li Tao1,2, Gao Yan Hua1, Lu Chun Fang1 and Abulimiti Yili1*
1State Key Laboratory Basis of Xinjiang Indigenous Medicinal Plants Resource Utilization, and the Key Laboratory of Chemistry of Plant Resources in Arid Regions, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, South Beijing Road, Urumqi, China
2University of the Chinese Academy of Sciences, Beijing, China
*Address for Correspondence: Abulimiti Yili, State Key Laboratory Basis of Xinjiang Indigenous Medicinal Plants Resource Utilization, and the Key Laboratory of Chemistry of Plant Resources in Arid Regions, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, 830011, South Beijing Road, Urumqi, China, Email: [email protected]
Azotobacter was selectively isolated and purified from the soil samples of Xinjiang Salt Lake Scenic spot, the fermentation technology of exopolysaccharides (EPS) by Azotobacter was optimized, and the antioxidant activity of exopolysaccharides (EPS) was studied. The bacteria were isolated and purified from the soil samples by the scribing method and the 16SrRNA gene was used for molecular identification. The carbon source, fermentation time, inoculation amount and pH of target bacteria in the exopolysaccharides (EPS) fermentation process were optimized through single-factor experiments and their antioxidant activity was measured. Eight types of Azotobacter were isolated and purified from the soil samples of Salt Lake scenic spot. Among them, As101, which showed 99.58% homology with Azotobacter salinestris, was selected as the target strain. Through single-factor experiments which used exopolysaccharides (EPS) yield and exopolysaccharides content as indexes, the optimal conditions for the As101 fermentation process were determined as follows: fermentation temperature 35, fermentation time 96h, pH 7 and mannitol as carbon source. Exopolysaccharides content from Azotobacter salinestris was 61.35% and the yield was 6.34 g/L. The results of the exopolysaccharides (EPS) antioxidant activity experiment under optimal conditions showed that As101 EPS had excellent scavenging ability against DPPH free radical, ABTS free radical and hydroxyl free radical, with IC50 values of 6.11 mg/ml, 2.42 mg/ml and 9.57 mg/ml, respectively. As101 with high yield and high exopolysaccharides content was isolated from saline soil in a special environment of Xinjiang, and the EPS obtained showed excellent antioxidant activity. The Azotobacter found in this study would provide the material basis for further opening up the adsorption of exopolysaccharides on heavy metals and the improvement of saline-alkali soil and contribute to further understanding of the structure and other activities of exopolysaccharides derived from Azotobacter.
Exopolysaccharides (EPS) are high-quality polymer that is excreted by microorganisms into the surrounding environment and mainly consists of sugar residues [1]. In recent years, researchers have found a variety of biological activities of microbial polysaccharides, whose antioxidant activity has been applied in food, cosmetics, and other fields [2]. Exopolysaccharide has a good curative effect in the treatment of gastric cancer, colon cancer and lung cancer. As immunoadjuvant drugs, the extracellular polysaccharide is mainly used to inhibit the occurrence, development and metastasis of tumors, improve the sensitivity of tumors to chemotherapy drugs, and improve the physical condition of patients. When used in combination with chemotherapeutic agents, exopolysaccharide has the effect of attenuating toxicity and enhancing efficacy. The combination of exopolysaccharides with other drugs in the treatment of chronic hepatitis B can improve the negative effect of hepatitis B virus markers and reduce the side effects of antiviral drugs. In addition, exopolysaccharides can be used to treat mycobacterium tuberculosis infections. Exopolysaccharides play the role of safe food additives and may become a good source of food-grade polysaccharides, which are widely used in thickening, stabilizing, emulsifying, gelling, and water holding of various foods. Since exopolysaccharides can interact with metal ions, exopolysaccharides can be used to remove metal ions from wastewater. This is widely used in wastewater, especially industrial wastewater containing heavy metals, and usually has good metal removal effects [3,4]. These products are classified as low-cost, non-toxic, naturally degradable compounds that can be used as effective alternatives to plant and algal products. In recent years, many studies have reported the antioxidant activity of exopolysaccharides, which can scavenge DPPH free radicals, superoxide anion free radicals and hydroxyl free radicals in vitro and also have the ability to resist lipid peroxidation. The antioxidant activity of exopolysaccharides is closely related to their band groups. In addition, exopolysaccharides with antioxidant activity also help to improve the body’s anti-aging, anti-fatigue, anti-tumor, and anti-radiation capabilities. Azotobacter is a kind of non-symbiotic diazotrophic bacteria, which occurs in neutral or slightly alkaline soil, including dust transported by air. Most of them exist in large numbers in the rhizosphereinterfoliar regions of plants. Azotobacter is known to produce large amounts of EPS, usually in the form of large mucous colonies when separated from soil habitats [5,6]. This exopolysaccharide can also protect the penetration of toxic metal ions into cells and nitrogen fixation in high oxygen concentration environments [7]. In addition, exopolysaccharides produced by Azotobacter also have various applications and activities [8-15]. Microbial polysaccharides possess some advantages compared to plant and animal polysaccharides [16]. Since microbial polysaccharides can be produced by fermentation, which has a short cycle, cost-effective, is not restricted by region and season, is easy to operate and has a stable supply, microbial polysaccharides have strong competitiveness and wide application prospect [17]. In conclusion, the abundant Azotobacter resources in Xinjiang saline-alkaline soil are advantageous resources for discovering and mining Azotobacter. Azotobacter EPS is currently applied in various fields, such as medicine, materials, functional food, environmental remediation, etc. The key issue of its application is to establish an efficient preparation process for EPS with high yield and high purity. Therefore, the research on the exopolysaccharides extraction process and antioxidant activity of Azotobacter As101 in this project provides technical support for the research and development of Azotobacter resources in Xinjiang.
Soil: Salt Lake City Scenic Spot, Urumqi, Xinjiang, China, collected on June 5, 2021. The soil samples were drilled vertically with a soil drill with an inner diameter of 6 cm by the five-point sampling method, and a soil depth of 1 cm - 20 cm was taken. After collection, the soil samples were stored at −4 ℃ and brought back to the laboratory for storage at -20 ℃.
Medium: Glucose 10.0 g, potassium dihydrogen phosphate 0.2 g, magnesium sulfate 0.2 g, sodium chloride 0.2 g, calcium sulfate 0.2 g, calcium carbonate 5.0 g, distilled water 1000 mL, 1 × 105 Pa sterilization 30 min; Nitrogen fixing medium: mannitol 20.0 g, potassium dihydrogen phosphate 0.2 g, potassium dihydrogen phosphate 0.8 g, magnesium sulfate 0.2 g, calcium sulfide 0.1 g, yeast paste 0.5 g, ferric chloride trace, sodium molybdate trace, distilled water 1000 mL, 1 × 105 Pa sterilization 20 min. The above medium has a pH of 7.0 - 7.2. Solid medium with 1.8% agar powder.
Isolation and purification of bacteria: Take 10 g soil sample, add 90 ml sterile water, shake well for 30 min, take supernatant for gradient dilution (10-1, 10-2, 10-3, 10-4, 10-5), take 100 µL diluted bacterial solution uniformly coated in Ashby solid medium, incubate in 37 ℃, 55% humidity incubator for 5-7 d, Single colonies with fast growth and obvious different morphology were selected, and the single colonies with the same morphology were obtained by continuous line purification for classification and identification.
Molecular identification of 16S rRNA gene: TIANGEN genomic DNA extraction kit (Cat. # DP302-02) was used to extract the DNA of each strain. DNA was used as a template after the detection and adjustment of the appropriate concentration. 16S rRNA universal primer 27F/1492R was used to amplify the target fragment, 27F: AGAGTTTGATCCTGGCTCAG; 1492R: GGTTACCTTGTT ACGACTT. The PCR amplification system was as follows: template 1.5 µL, upstream and downstream primers 1 µL each, 2*Taq PCR Green Mix 25 µL, sterile water supplement to the total volume 50 µL. The PCR amplification condition was 95 ℃ for 5 min.94 ℃ for 50 s; 54 ℃ for 30s; A total of 30 cycles at 72 ℃ for 30s; 72 ℃ for 7 min. The PCR-amplified products were sent to Shanghai Biotech for sequencing. After the strain 16S rRNA gene sequence was sequenced, the similarity was compared on the NCBI website and the strain registration number was submitted to the GenBank database.
EPS extraction method: The fermentation liquid was concentrated to 1/3 with a rotary evaporator and precipitated with 4 times the volume of ice ethanol. The liquid was placed at 4 ℃ for 12h, centrifuged, and precipitated. The precipitates were dissolved in water and deproteinized 3 times with savage reagent. After deproteinization, the sample was dialyzed for 48 hours to remove salt and small molecular impurities. Freeze-dried and weighed. Repeat three times. The content of sugar was determined by the anthrone sulfuric acid method.
Determination of polysaccharide content: According to standard DB12/T884-2019, the “anthrone sulfuric acid method” was used to determine the polysaccharide content in the extracellular polysaccharide of Azotobacter As101 with glucose standard as the reference substance [18].
Exopolysaccharides yield: The final weight of crude polysaccharides per liter of fermentation broth was obtained by the EPS extraction method.
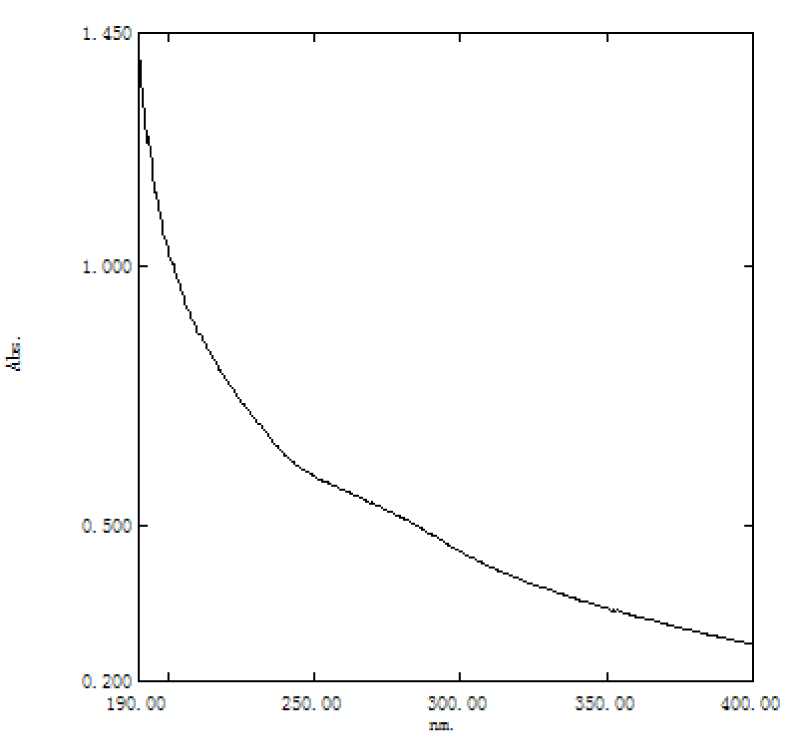
Characterization and purity identification of EPS: The infrared spectrum of extracellular polysaccharide of Azotobacter As101 was determined by the potassium bromide tablet method. The polysaccharides were thoroughly mixed with dried potassium bromide and ground into fine powder particles. Then the scanning analysis was carried out by infrared spectrophotometer (Thermo, Nicolet 6700) in the range of 400 cm-1 – 4000 cm-1; An aqueous solution of azotobacter As101 extracellular polysaccharide with a concentration of 2 mg/ml was prepared and the full wavelength scanning absorbance in the range of 200 nm – 400 nm was measured by ultraviolet spectrophotometer.
Effect of different fermentation times on EPS sugar content: Under the conditions of fermentation temperature of 35 ℃, pH value of 7, mannitol as carbon source and inoculation amount of 5%, the fermentation time of 24h, 48h, 72h, 96h, and 120h were selected to investigate the influence of extraction time factors on sugar content; Effects of different pH values on EPS sugar content: Under the conditions of fermentation temperature of 35 ℃, fermentation time of 120h, mannitol as carbon source and inoculation amount of 5%, pH values of 7,8,9,10,11,12 were selected to investigate the influence of pH factors on sugar content; Effects of different carbon sources on EPS sugar content: Under the conditions of fermentation temperature 35 ℃, fermentation time 120h, pH 7 and inoculation amount 5%, different carbon sources such as lactose, sucrose, mannitol, mannose-glucose were selected to carry out the influence of carbon sources on sugar content.
Determination of the antioxidant activity of EPS: DPPH+, ABTS+, and OH+ scavenging capacity were measured respectively [19]. DPPH free radical scavenging ability: EPS samples were configured with five concentrations of 10 mg/ml, 5 mg/ml, 2.5 mg/ml, 1.25 mg/ml, and 0.625 mg/ml, and 100 µL of each concentration was added to the 96-well plate. Then 100 µL DPPH free radical (0.2 mmol/L) was added to avoid light for 30 min and the absorbance was measured at 517 nm using an enzyme marker. Repeat three times. The DPPH free radical scavenging rate was calculated as follows:
Where, Ai, Aj and A0 are the absorbances of the sample group, control group and blank group respectively.
ABTS free radical scavenging ability: EPS samples were respectively configured with five concentrations of 10 mg/ml, 5 mg/ml, 2.5 mg/ml, 1.25 mg/ml, and 0.625 mg/ml and each concentration was added to the 96-well plate with 50 µL of ABTS free radical solution and 150 µL of ABTS free radical solution. The reaction was carried out at room temperature for 10 min, and the absorbance was measured at 734 nm using an enzyme label instrument. The absorbance was repeated three times. The ABTS free radical scavenging rate was calculated as follows:
Where, Ai, Aj and A0 are the absorbances of the sample group, control group and blank group respectively.
Hydroxyl free radical scavenging ability: EPS samples were respectively configured with five concentrations of 10 mg/ml, 5 mg/ml, 2.5 mg/ml, 1.25 mg/ml, and 0.625 mg/ml, and 50 µL of each concentration was added into 50 µL of prepared ferrous sulfate (6 mmol/l) solution. 50 µL salicylic acid-ethanol (6 mmol/l) solution was mixed, then 50 µL 0.1% hydrogen peroxide solution was added, and placed at 37 ℃ for 30 min. The absorbance was measured at 510 nm using an enzyme spectrometer and repeated 3 times. The hydroxyl radical scavenging rate was calculated as follows:
Where, Ai, Aj and A0 are the absorbances of the sample group, control group and blank group respectively.
Eight strains were isolated from soil by Ashby medium. Among them, Pa3, Ec5-1, As101, and other bacteria were sequenced by 16S rRNA and compared to belong to the genus Pantoea agglomerans (Pa3), Enterobacter (Ec5-1), Azotobacter salinestris (As101), respectively. The homology of strain Pa3 and Pantoea agglomerans strain S19_P A1R was 99.79%, and the similarity of other strains ranged from 99.14% to 99.79%. The homology between strain As101 and Azotobacter salinestris was 99.58% (Table 1). Therefore, we chose As101 as the target strain for the following study.
| Table 1: The sequence result and homology analysis for 8 bacterium strains. | |||
| Strain number | Highest similarity strain | Homology% | Registered number |
| Pa 3 | Pantoea agglomerans strain S19_PA1R | 99.79% | OK445502 |
| Ec 5-1 | Enterobacter cloacae subsp. dissolves strain M354 | 99.79% | OK445503 |
| E 9-2 | Enterobacter sp. strain KJK 2.1 | 99.79% | OK445504 |
| Eh 26 | Enterobacter hormaechei | 99.59% | OK445506 |
| Ehs 29 | Enterobacter hormaechei subsp. Hoffmannii | 99.66% | OK445507 |
| El 31 | Enterobacter ludwigii | 99.14% | OK445508 |
| Pa 34 | Pantoea agglomerans | 99.93% | OK445509 |
| As101 | Azotobacter salinestris | 99.58% | OK445517 |
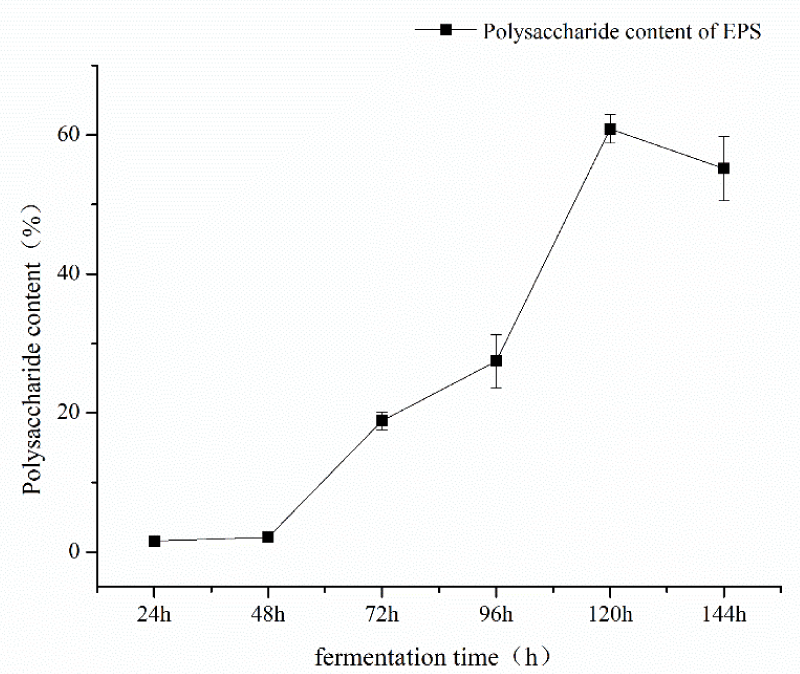
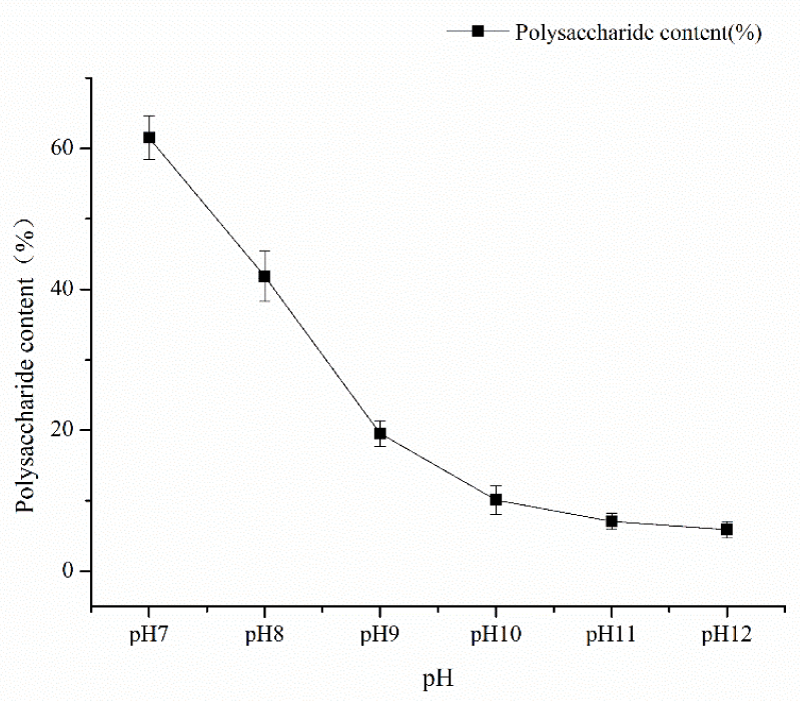
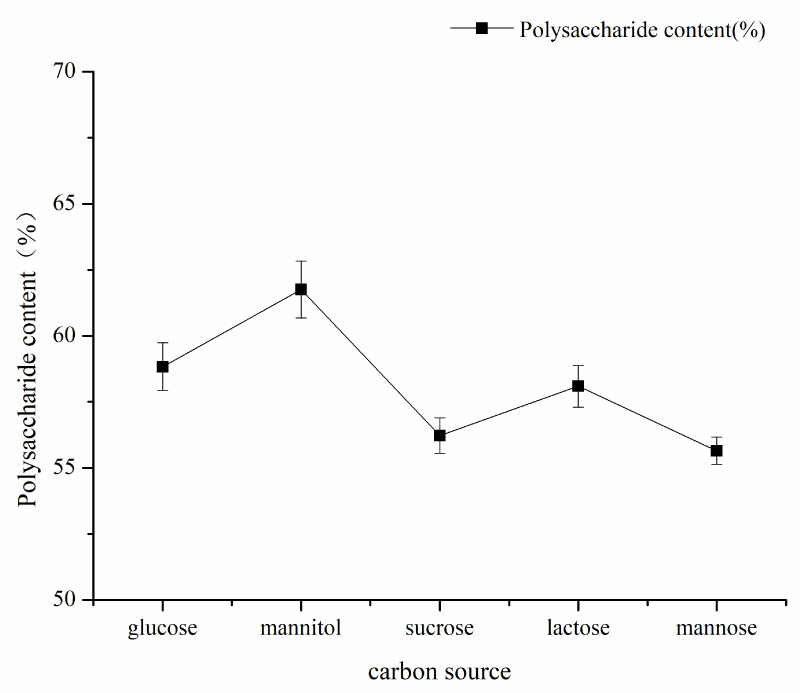
Single factor experiment of fermentation time: under the conditions of fermentation temperature of 35 ℃, pH 7, mannitol as carbon source and inoculation amount of 5%, the fermentation time was 24h, 48h, 72h, 96h, and 120h respectively. The influence of the fermentation time factor on sugar content was shown in Figure 1. As can be seen from Figure 1, the fermentation time of polysaccharide yield and content showed an increasing trend from 48h to 120h and the fermentation time reached a large value at 120h, and then tended to wane. The results showed that fermentation time could increase the content of polysaccharides within a certain range. Therefore, the optimal fermentation time was 120h considering the time saving of polysaccharide content; single factor pH test: under the conditions of fermentation time of 120, the temperature of 35 ℃, mannitol as carbon source, and inoculation amount of 5%, pH values of 7, 8, 9, 10, 11 and 12 respectively, the influence of pH factor on sugar content is shown in Figure 2. As can be seen from Figure 2, pH value had a great impact on the production of Azotobacter As101. The polysaccharide content was the highest under the pH7 condition, and the more alkaline the pH condition is, the more inhibiting the production of metabolites. Therefore, the optimal fermentation pH value of Azotobacter As101 is 7; single factor test with different carbon sources: under the conditions of fermentation time of 120, a temperature of 35 ℃, pH value of 7, and inoculation amount of 5%, five different carbon sources were selected as glucose, mannitol, sucrose, lactose and mannose-and the influence of different carbon source factors on sugar content was shown in Figure 3. It can be seen from Figure 3 that different carbon sources had little influence on the sugar content of Azotobacter As101, among which the original formula mannitol in the nitrogen-fixing medium had the best sugar content when the carbon source was used. Therefore, the carbon source is mannitol as the optimal medium carbon source.
Figure 1: Effect of fermentation time on polysaccharide yield and content.
Figure 2: Effect of pH value on polysaccharide yield and content.
Figure 3: Effect of different carbon sources on the yield and content of Polysaccharides.
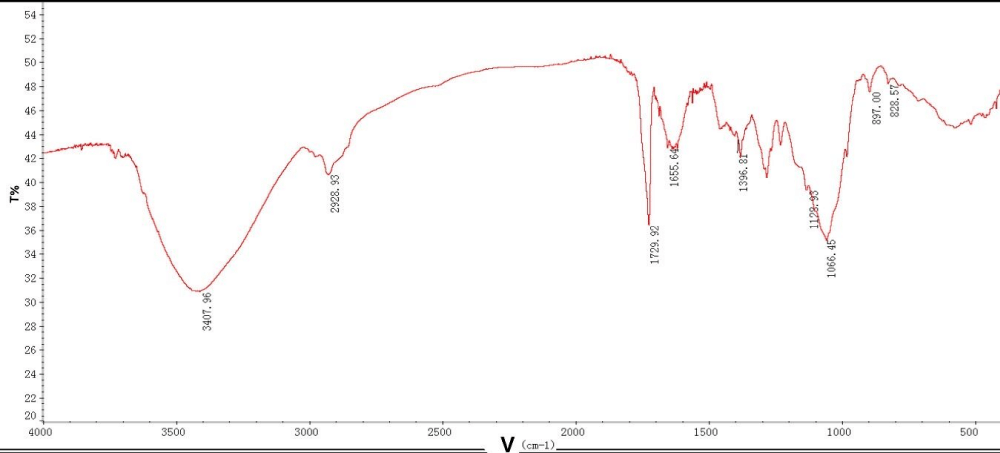
In the infrared spectrum, the wide and large absorption peak near 3407 cm-1 is caused by O-H stretching vibration. The absorption peak near 2920 cm-1 is caused by C-H bending vibration. The absorption at about 1729 cm-1 is due to the asymmetric tensile vibration of the C = O absorption peak, indicating the presence of uronic acid in EPS, which is consistent with the result of other chemical cocompanionna lysis. The absorption peaks in the 1200 cm-1 - 1000 cm-1 range were attributed to C-O-C stretching vibrations iglycophorinochain skeleton, indicating the possible present ran-typen type sugar rings. In the near and 830 cm-1, 890 1 of absorption peak shows that it probably contains both configurations betatconfigurationne ns of the glycosidic bond. Among the above absorption peaks, the hydroxyl absorption peak of 3407 cm-1, the carbonyl absorption peak of 1729 cm-1, and the sugar skeleton absorption peak arise characteristic absorption peaks of exopolysaccharides.
EPS showed no absorption peak near 260 nm - 280 nm, indicating that it did not contain proteins, peptides and nucleic acids and was completely deproteinized Table 2, Figures 4,5.
| Table 2: IR spectrum of extracellular polysaccharide from azotobacter As101. | |
| USP-P Absorption wavelength(cm-1) | Radical |
| 3407 | μ(OH) |
| 2928 | μ(CH) |
| 1729 | μ(C=O) |
| 1655 | μ(H2O) |
| 1396 | μ(CH3) |
| 1128 | μ(C-O-C) |
| 1066 | μ(C-O-C) |
| 897 | >β configuration |
| 828 | a configuration |
Figure 4: IR spectrum of extracellular polysaccharide from azotobacter As101.
Figure 5: UV spectrum of extracellular polysaccharide from azotobacter As101.
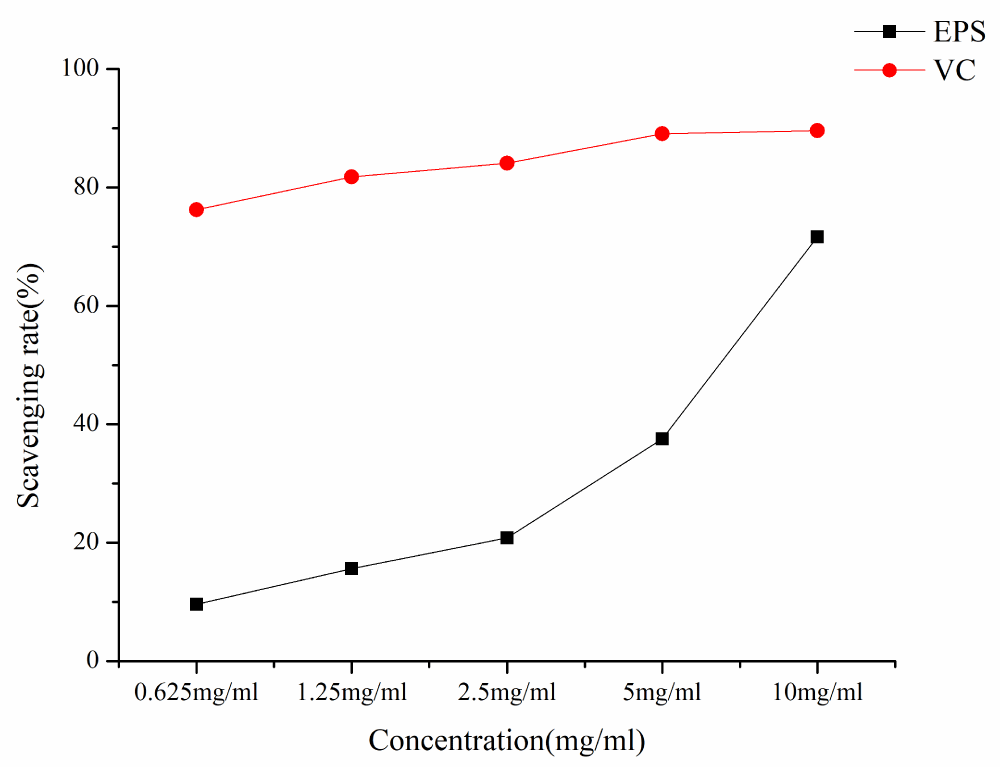
As shown in Figure 6, EPS has certain scavenging activities against DPPH·. The DPPH scavenging ability of EPS and VC increased with the increase of EPS concentration in a concentration-dependent manner. At the lowest concentration of 0.625 mg/ml, the DPPH· clearance rate was 9.59%, at the concentration of 10 mg/ml, the DPPH· clearance rate was 71.65%, and IC50 was 6.11 mg/ml.
Figure 6: DPPH radical scavenging rates of different samples.
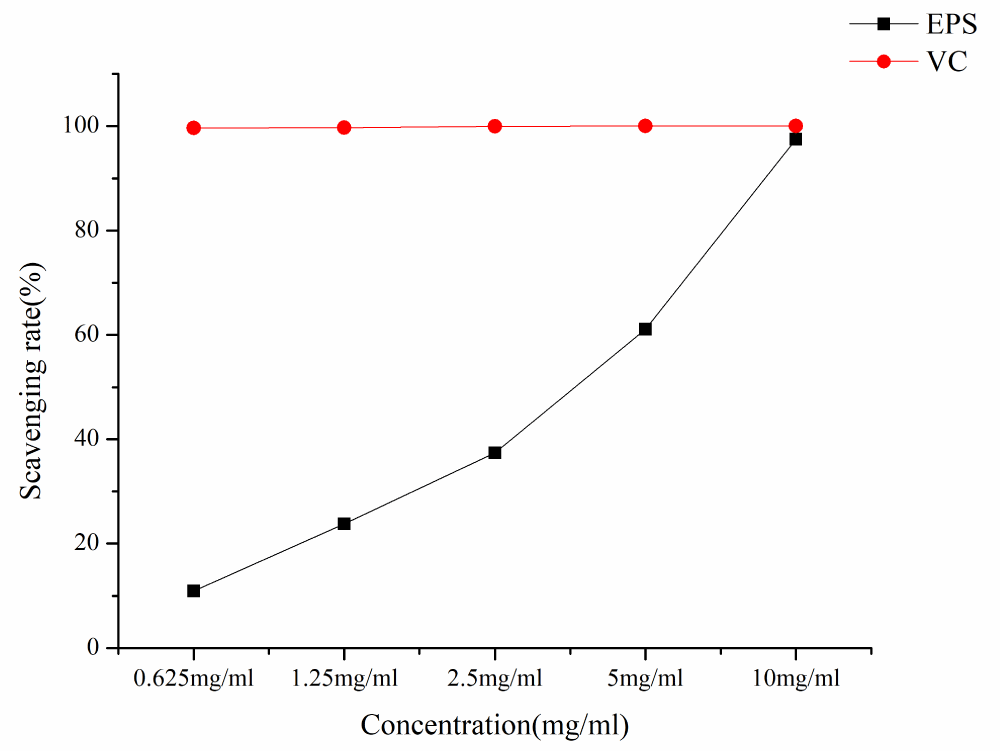
As can be seen from Figure 7, the ABTS radical scavenging ability of EPS and VC increased with the increase of EPS concentration in a concentration-dependent manner. At the lowest concentration of 0.625 mg/mL ABTS· scavenging activity was 10.96%, at the concentration of 10mg/ml ABTS· clearance rate is 97.45%, and IC50 is 2.42 mg/ml.
Figure 7: ABTS radical scavenging rates of different samples.
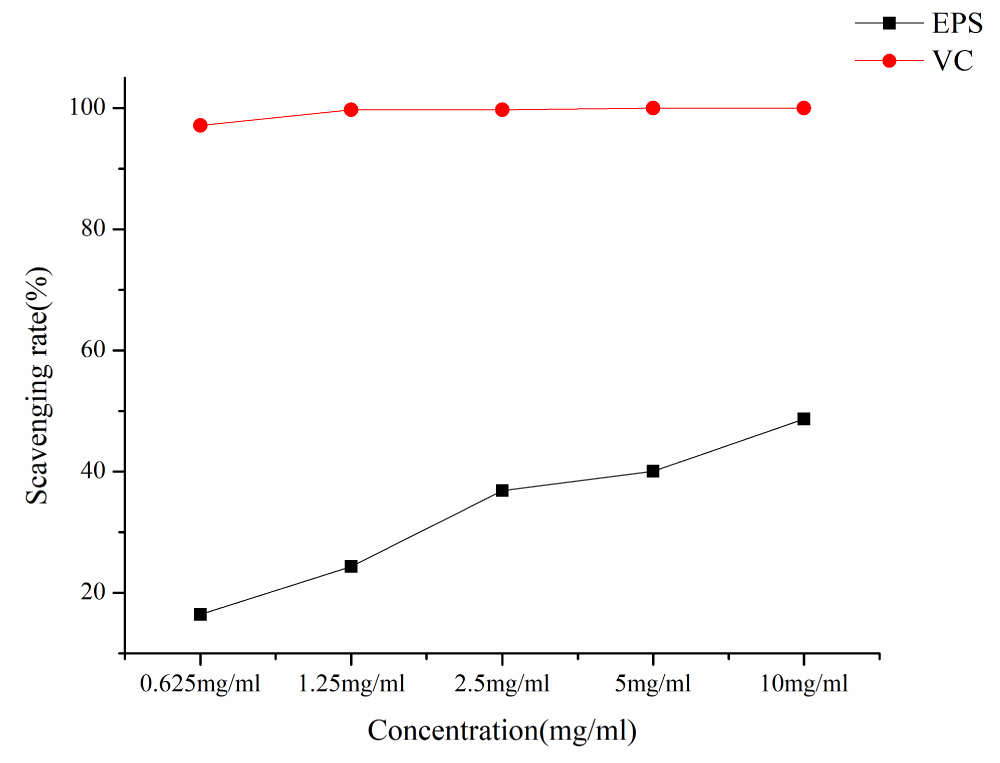
As shown in Figure 8, EPS has a certain scavenging effect on hydroxyl free radicals. The hydroxyl radical scavenging ability of EPS and VC increased with the increase of EPS concentration in a concentration-dependent manner. At the lowest concentration of 0.625 mg/ml, the hydroxyl radical scavenging rate was 16.42%, at the concentration of 10 mg/ml, the hydroxyl radical scavenging rate was 48.68% and the IC50 was 9.57 mg/ml.
Figure 8: Hydroxyl radical scavenging rates of different samples.
In this work, Azotobacter As101 (Azotobacter salinestris) w as isolated and purified from the saline soil in the unique soil environment of Xinjiang. The content of extracellular polysaccharide (EPS) was used as the index to optimize the fermentation conditions. The optimized fermentation temperature is 35 ℃ and the fermentation time is 120h. The final exopolysaccharide content is 61.35% and the yield is 6.34 g/L when the pH value is 7, mannitol is the carbon source and the inoculation amount is 5%. This result was higher than the polysaccharides yield of 5.05 g/L obtained by Jia Yuxiang [12] for Rhizobium NG10. Satish [13] studied the fermentation process of Azotobacterindicus and obtained a polysaccharide yield of 6.10 g/L. Lin [20] studied Lactobacillus helveticus BCRC14030, L. helveticus BCRC14076, and Streptococcus thermophilus BCRC14085, and finally obtained the highest polysaccharide yield of 0.73 g/L. The extracellular polysaccharide yield of Marine actinomycetes studied by CAI Huinong, et al. [21] was 4.58 g/L. It can be shown that the isolated azotobacter As101EPS has relatively high extracellular polysaccharide yield and polysaccharide content compared with other bacteria. Some literature also reported that the exopolysaccharides produced by fungi were high [22], but the sugar content was relatively low, which may be caused by different types of microorganisms, which should be further studied. Azotobacter As101 EPS has strong antioxidant activity. The trend of the free radical scavenging ability of VC was increased in activity analysis. Although the scavenging activity of VC solution on the three kinds of free radicals was positively correlated with the mass concentration and the scavenging activity was higher than that of the exopolysaccharides of Azotobacter As101, the variation trend of VC solution increased slowly with the concentration, while the scavenging ability of exopolysaccharides of Azotobacter As101 against DPPH· and ABTS· free radicals showed a “strong inflection point” and strong linear characteristics with the increase of concentration, especially as shown in Figure 6. The scavenging abilities of Azotobacter As101 ((Azotobacter salinestris) e xopolysaccharide against DPPH·, ABTS· free radicals are shown in Figure 7. When the concentration of ABTS· scavenging capacity of azotobacter As101 exocellular polysaccharide reached 10 mg/ml, it reached 97.45%, which was close to the ABTS· scavenging rate of VC, indicating that the higher the concentration, the stronger the antioxidant capacity. The results were higher than those obtained by Wang Chunwei [23], He Yatong [24], and Fan Yijun [25], and lower than those obtained by Azotobactor As101 exopolysaccharide. The relationship between the better antioxidant capacity of EPS and its structure and composition needs further study. This experiment encountered some problems in the fermentation process research. Because the formula of the fermentation medium contains sugar elements, the fermentation conditions must be consistent each time to complete the process of alcohol precipitation, deproteinization, dialysis and freeze-drying of polysaccharides, which was repeated three times and required a long time. It was found in the experiment that the fermentation conditions of azotobacter As101 were mainly affected by time and pH, but the influence of different carbon sources on EPS was not obvious. The influence of other factors such as inorganic salts and nitrogen sources on exopolysaccharides can be further should be studied.
Although a variety of physiological functions of exopolysaccharides have been reported, a few are still under investigation. In particular, the exopolysaccharides of Azotobacter salinestris in the genus of Azotoacter have not been studied. In this paper, Azotobacter salinestris As101 (Azotobacter salinestris), which was isolated from the saline soil in the special environment of Xinjiang, laid a foundation for the exploration of environmental restoration functions such as salt adsorption and heavy metal adsorption of microbial polysaccharides to develop the microbial resources in the extreme environment of Xinjiang and study the structure analysis and activity of microbial polysaccharides. The structure, composition, activity, and application of exopolysaccharides from Azotobacter As101 should be studied.
Author contributions
This study was conceived by A.Y. and N.H.X.; conducted by P.P., N.L.T., G.Y.H., and L.C.F and overseen and directed by A.Y. and N.H.X. All authors participated in the research and in the article preparation. All authors have read and agreed to the published version of the manuscript.
Funding: This research was supported by the West Light Foundation of the Chinese Academy of Sciences (2021-XBQNXZ-026).
- Aleem A, Isar J, Malik A. Impact of long-term application of industrial wastewater on the emergence of resistance traits in Azotobacter chroococcum isolated from rhizospheric soil. Bioresour Technol. 2003 Jan;86(1):7-13. doi: 10.1016/s0960-8524(02)00134-7. PMID: 12421001.
- Ding L. Isolation, purification and antioxidant activity of exopolysaccharides from a bacterial strain. Northwestern University. 2021.
- Binbin L, Qing S, Bo M .Application of exopolysaccharide directionally synthesized by Xanthomonas campestris as the green selective depressant for the clean flotation of talc: Statistical optimization and mechanism analysis. Journal of Cleaner Production. 2023; 383: 135381.
- Dina H. Antioxidant and antimicrobial activities of exopolysaccharides produced by a novel Aspergillus sp. DHE6 under optimized submerged fermentation conditions. Biocatalysis and Agricultural Biotechnology. 2021; 36: 102150.
- Delattre C, Pierre G, Laroche C, Michaud P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol Adv. 2016 Nov 15;34(7):1159-1179. doi: 10.1016/j.biotechadv.2016.08.001. Epub 2016 Aug 12. PMID: 27530696.
- Gauri SS, Mandal SM, Mondal KC, Dey S, Pati BR. Enhanced production and partial characterization of an extracellular polysaccharide from newly isolated Azotobacter sp. SSB81. Bioresour Technol. 2009 Sep;100(18):4240-3. doi: 10.1016/j.biortech.2009.03.064. Epub 2009 Apr 28. PMID: 19403304.
- Rana S, Upadhyay LSB. Microbial exopolysaccharides: Synthesis pathways, types and their commercial applications. Int J Biol Macromol. 2020 Aug 15;157:577-583. doi: 10.1016/j.ijbiomac.2020.04.084. Epub 2020 Apr 15. PMID: 32304790.
- Morten KDD, Maaike B, Emil JZ.Genetic potential for exopolysaccharide synthesis in activated sludge bacteria uncovered by genome-resolved metagenomics. Water Research. 2022; 119485.
- Rodríguez C, Medici M, Rodríguez AV, Mozzi F, Font de Valdez G. Prevention of chronic gastritis by fermented milks made with exopolysaccharide-producing Streptococcus thermophilus strains. J Dairy Sci. 2009 Jun;92(6):2423-34. doi: 10.3168/jds.2008-1724. PMID: 19447974.
- Cheng HB, Han Y, Ren S. Isolation, Purification and Anti S180 Sarcoma of Extracellular Polysaccharides of Rhizobium sp. N613. Chinese Journal of Pharmacy, 2008; 43(4): 5.
- Zhang JG. Study on the Synthesis of Polyhydroxyalkanoic Acid and Polysaccharides by Rhizobium SP 1201. Sichuan Agricultural University. 2005.
- Ia YX, Geng XQ, Huang ZM. Fermentation optimization and anti-tumor activity of polysaccharides from Rhizobium sp.NG10. Food and Fermentation Industries. 2019; 45:5.
- Patil SV, Salunkhe RB, Patil CD, Patil DM, Salunke BK. Bioflocculant exopolysaccharide production by Azotobacter indicus using flower extract of Madhuca latifolia L. Appl Biochem Biotechnol. 2010 Oct;162(4):1095-108. doi: 10.1007/s12010-009-8820-8. Epub 2009 Nov 18. PMID: 19921493.
- Sofía DC, María CO, Aparna B. Biological properties of exopolysaccharides produced by Bacillus spp. Microbiological Research. 2023; 268: 127276.
- Zhuang Z, Yang G, Zhuang L. Exopolysaccharides matrix affects the process of extracellular electron transfer in electroactive biofilm. Sci Total Environ. 2022 Feb 1;806(Pt 3):150713. doi: 10.1016/j.scitotenv.2021.150713. Epub 2021 Oct 2. PMID: 34606863.
- Sun L, Cheng L, Ma Y, Lei P, Wang R, Gu Y, Li S, Zhang F, Xu H. Exopolysaccharides from Pantoea alhagi NX-11 specifically improve its root colonization and rice salt resistance. Int J Biol Macromol. 2022 Jun 1;209(Pt A):396-404. doi: 10.1016/j.ijbiomac.2022.04.015. Epub 2022 Apr 9. PMID: 35413311.
- Xiu AH. Preparation structure analysis and biological functions study for an extracellular polysaccharide produced by Agrobacterium sp. ZX09. Nanjing University of Technology. 2011.
- Yituxun A, Paerhati R, Gao YH. Optimization of Extraction Process and Structural Characterization of Polysaccharides from Fritillaria ussuriensis. Chinese pharmacy, 2020; 31(03): 276-281.
- Qianwen N, Junjun L, Maria CM. Selenium and flavonoids in selenium-enriched Tartary buckwheat roasted grain tea: Their distribution and correlation to antioxidant activity. LWT. 2022; 170:114047.
- Lin T, Chien M. Exopolysaccharides production as affected by lactic acid bacteria and fermentation time. Food Chemistry. 2007;100(4):1419-1423.
- Cai HN, Guo CH, Chen JF. A study on the fermentation technology for a marine actinomyces extracellular polysaccharide. Marine Sciences.2003; 75-80.
- LI CZ.Screening of Hericium erinaceus Strains with high active polysaccharide productivity and research on its metabolites harvested at different developmental stages. Northwestern University.2012.
- Wang CW, Bai Y. Study on Physicochemical Properties and Antioxidant Properties of Kefir Extracellular Polysaccharide. Food and Fermentation Industries.2022; 48(21):104-110.
- He YT, Ma L, Li W. Study on Extraction, Purification and Antioxidant Activity of Polysaccharides from Eucalyptus citriodora Resin. Applied chemical industry. 2022; 008:051.
- Fan Y, He X, Zhou S, Luo A, He T, Chun Z. Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int J Biol Macromol. 2009 Aug 1;45(2):169-73. doi: 10.1016/j.ijbiomac.2009.04.019. Epub 2009 May 3. PMID: 19414030.