More Information
Submitted: April 06, 2023 | Approved: April 14, 2023 | Published: April 17, 2023
How to cite this article: Simão-Silva EB, Serrano NFG, de Medeiros MPC, Boareto-Mendes AJ, Galdino JF, et al. Nuclear science and magnetic carbon: a promising way from a chemical method to detect and fight cancer and tumors/neoplasms. Ann Adv Chem. 2023; 7: 047-050.
DOI: 10.29328/journal.aac.1001042
Copyright License: © 2023 Simão-Silva EB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Biosensors; Detectors; Radioactive iodine; Cancer; Magnetic carbon/graphite; Drug carriers; Radioactive Boron; BNCT technique; Nanostructured carbon; Antibody-antigen interaction
Nuclear science and magnetic carbon: a promising way from a chemical method to detect and fight cancer and tumors/neoplasms
EB Simão-Silva1, NFG Serrano2, MPC de Medeiros1, AJ Boareto-Mendes1, JF Galdino1 and FM Araujo-Moreira1*
1Military Institute of Engineering/IME, Praça General Tibúrcio 80, Urca- Rio de Janeiro 22290-270 RJ, Brazil
2Federal University of São Carlos/UFSCar, Department of Morphology and Pathology; Laboratory of Inflammation and Infectious Diseases, PO Box 676, São Carlos 13565-905 SP, Brazil
*Address for Correspondence: FM Araujo-Moreira, Military Institute of Engineering /IME, Department of Science and Technology; Brazilian Army, Praça General Tibúrcio 80, Urca, Rio de Janeiro, RJ 22290-270, Brazil, Email: [email protected]
In 2005 we reported for the first time on a chemical route aiming to synthesize stable magnetic carbon/graphite. By using the Nuclear Magnetic Resonance (NMR) technique we have verified that its magnetism is an intrinsic property of this synthesized material and not originated from ferromagnetic impurities of any kind. Through direct measurement of the local magnetic field using Carbon-13, we have concluded that its magnetism originated from defects in the structure. From its biocompatibility, we have been working on the use of magnetic carbon/graphite to deliver many compounds aiming to fight different diseases. Despite all the scientific and technological advances of the present day, cancer is a multifactorial and difficult-to-treat disease, killing hundreds of thousands of people a year worldwide. Therefore, the development of a new and efficient drug delivery system to fight cancer – among other diseases - is as important as the discovery of a novel active molecule. In this review of our own work, we show the drug delivery system named MAGUS® (an acronym for Magnetic Graphite Universal System) we have built based on nanostructured magnetic carbon/graphite. This is an innovative and promising system composed of a biocompatible nanostructured particle of magnetic carbon/graphite functionalized with different molecules and materials. MAGUS®, depending on what we link to its structure, is so versatile and can be used to detect a wide range of specimens, from tumors and cancers to chemical and biological agents used as non-conventional weapons. That is why we call it universal. In the present work, MAGUS® will be acting as a biosensor, where the magnetic carbon/graphite is functionalized with radioactive particles of Iodine-131 and antibodies of different types of cancer. Then, by focusing on both the antigen-antibody interaction and the spatial guiding through an external magnetic field we are providing our drug delivery system a double way to detect and reach just the target. Based on these strategies, the functionalized magnetic carbon/graphite will reach only the neoplasm and not the surrounding healthy cells around. In a general view, it means that we are giving specificity to the MAGUS® drug delivery system as a pioneering and effective way to detect and treat cancers. We are also working on this unprecedented and efficient drug delivery system using the principles of Boron Neutron Capture Therapy (BNCT) with Boron-10 instead of Iodine-131. BNCT technique uses neutrons as the external source and is frequently employed to treat specific tumors that are radio resistant or very difficult to kill using conventional radiation therapy. In summary, we show here for the first time that our Magnetic Graphite Universal System associated with nuclear techniques can be successfully used as a biosensor to detect and fight cancers and tumors with powerful features that conventional delivery drug systems and other treatments do not have at all.
Throughout history, countless technological advances, originally intended for the development of military products and systems, have spilled over to other sectors generating disruptive innovations with enormous benefits for society. Particularly in the twentieth century, sophisticated research of military interest boosted innovations and the economic growth of pioneer countries. One of those advances was derived from nuclear science and engineering. Today, the so-called fourth industrial revolution is transforming the way people relate, work and enjoy their leisure and rest hours. On a broader spectrum, it is affecting economic growth, development, security and sovereignty of countries, international relations and the nature of warlike conflicts among other areas. Unlike its predecessors, which are based on disruptive innovations in specific areas, the fourth industrial revolution develops from the confluence of innovations that have occurred in various areas. Physics comprises incessant and surprising advances in new materials, sensors, nanotechnology, microelectronics and physical infrastructure of information and communication technologies; communication protocols, and algorithms used in a wide range of applications. Genetic algorithms, artificial neural networks, learning techniques, genetic sequencing, bioprinting, and drone swarms, are some of the lines of research inspired by such studies [1]. Recently, artificial intelligence (AI) through ChatGPT (or GPT-3, Generative Pre-Trained Transformer) by Open IA has started a worldwide scientific, technological, and social revolution in almost all possible aspects, by using 175 billion parameters. Its new generation, called GTP-4, is planned to use amazing 100 trillion parameters [2]. Scientists are considering that a further version, called GTP-5 (which will be available by the end of 2024) will have complete human capabilities.
Amazingly, despite all these incredible advances, cancer is a difficult-to-treat disease associated with a negative prognosis (depending on the stage of detection), that kills hundreds of thousands of people a year worldwide. Each year, the American Cancer Society estimates the number of new cancer cases and deaths in the United States and compiles the most recent data on population-based cancer occurrence and outcomes. Worldwide, cancer is a leading cause of death, accounting for nearly 10 million deaths in 2020, or nearly one in six deaths [3,4]. In 2022, 1,918,030 new cancer cases and 609,360 cancer deaths were projected to occur just in the United States, including approximately 350 deaths per day from lung cancer, the leading cause of cancer death. Even today, most methods to treat that disease are based on ionizing radiation (like gamma), or in some medicines, both methods result in broad unwanted side effects. They have no specificity and reach not only cancer but other parts of the human body. Unquestionably, both methods have saved hundreds of thousands of lives, however, cancer is still killing too many people worldwide. Surely in both, due to a high dose in the tumor neighbor tissues or due to a lower dose insufficient to kill cancer, it is highly desired to develop new and more effective methods to specifically fight it.
Developing MAGUS®
Taking this scenario into consideration we have been working on the use of magnetic carbon/graphite to deliver different compounds to fight many diseases. We know that the development of a new and efficient drug delivery system is as important as the discovery of a novel active molecule. Thus, based on nanostructured magnetic carbon/graphite we have built a drug delivery system named MAGUS®, which is an acronym for Magnetic Graphite Universal System. We have assembled this innovative and promising system as a biosensor composed of a biocompatible carbon particle functionalized with different molecules simultaneously. We reported how to obtain this magnetic carbon/graphite in 2005 and 2006 [5,6] by following an inexpensive chemical route consisting of a controlled chemical etching on the graphite structure, performed by a redox reaction in a closed system between pure carbon/graphite and copper oxide (CuO). It allows getting macroscopic amounts of magnetic carbon/graphite stable at room temperature and even above. X-ray diffraction measurements suggest that magnetic carbon/graphite could be represented by the coexistence of a matrix of pristine graphite and a foamy-like carbon/graphitic structure compressed along the c-axis. At T = 300 K, the saturation magnetic moment, the coercive field, and the remnant magnetization are 0.25 emu/g, 350 Oe, and 0.04 emu/g, respectively. Besides the phase transition at 300 K, it is possible to observe a low-temperature anomaly in the dependence of the zero-field-cooled magnetization in samples with an average granular size L of about 10nm. We have attributed it to the manifestation of the side effects below the quantum temperature TL∝ℏ2/L2. This behavior is well-fitted by a periodic function proportional to the bulk magnetization and the thermal De Broglie wavelength [7]. Related to that behavior, we have proposed a theoretical interpretation for both intragranular and intergranular contributions based, respectively, on super-exchange interaction between defects-induced localized spins in a single grain and proximity-mediated interaction between grains through the barriers created by thin layers of non-magnetic carbon/graphite [7]. In 2015, we experimentally confirmed that magnetism in carbon/graphite originates from defects in the structure (and not from ferromagnetic impurities of any type) from direct measurement of the local magnetic field using Carbon-13 Nuclear Magnetic Resonance (NMR) associated with the numerical results obtained from DFT (Density-Functional Theory) calculations. These experiments allowed us, for the first time, to directly evaluate the local hyperfine magnetic field in magnetic carbon/graphite samples corroborating the intrinsic and true nature of the magnetism. A comparison of the experimental hyperfine fields to DFT calculations showed reasonable agreement, supporting the view that magnetism originates from various defects in the material structure [8,9].
Developing MAGUS® associated to a conventional drug (Ibuprofen®)
We have verified the efficiency of this new drug delivery system by developing a magnetic bio-hybrid system from the assembly of the biopolymer alginate and magnetic carbon/graphite [9]. In this case, we have nanostructured the magnetic carbon/graphite particles as a nanofluid [10,11]. The drug Ibuprofen® (IBU) intercalated in a Mg–Al Layered Double Hydroxide (LDH) was chosen as a model of a drug delivery system to be incorporated as a third component of the magnetic bio-nanocomposite drug delivery system. The IBU was incorporated either as the pure drug or as the LDH–IBU intercalation compound and processed as beads or films for application as drug release systems. The presence of magnetic carbon/graphite nanoparticles improved the physical and mechanical properties of the resulting bionanocomposites, decreasing the speed of drug delivery due to the protective effect as a physical barrier against water absorption into the beads. The control on the release rate was specially improved when the drug was incorporated as the LDH–IBU intercalation compound, this fact was attributed to the additional physical barrier afforded by the inorganic layered host solid. These bio-nanocomposite systems could be stimulated by an external magnetic field as well, enhancing the levels of the released IBU, which would be advantageous to modulate the dose of the released drug when required [12].
Developing MAGUS® associated with radioactive particles
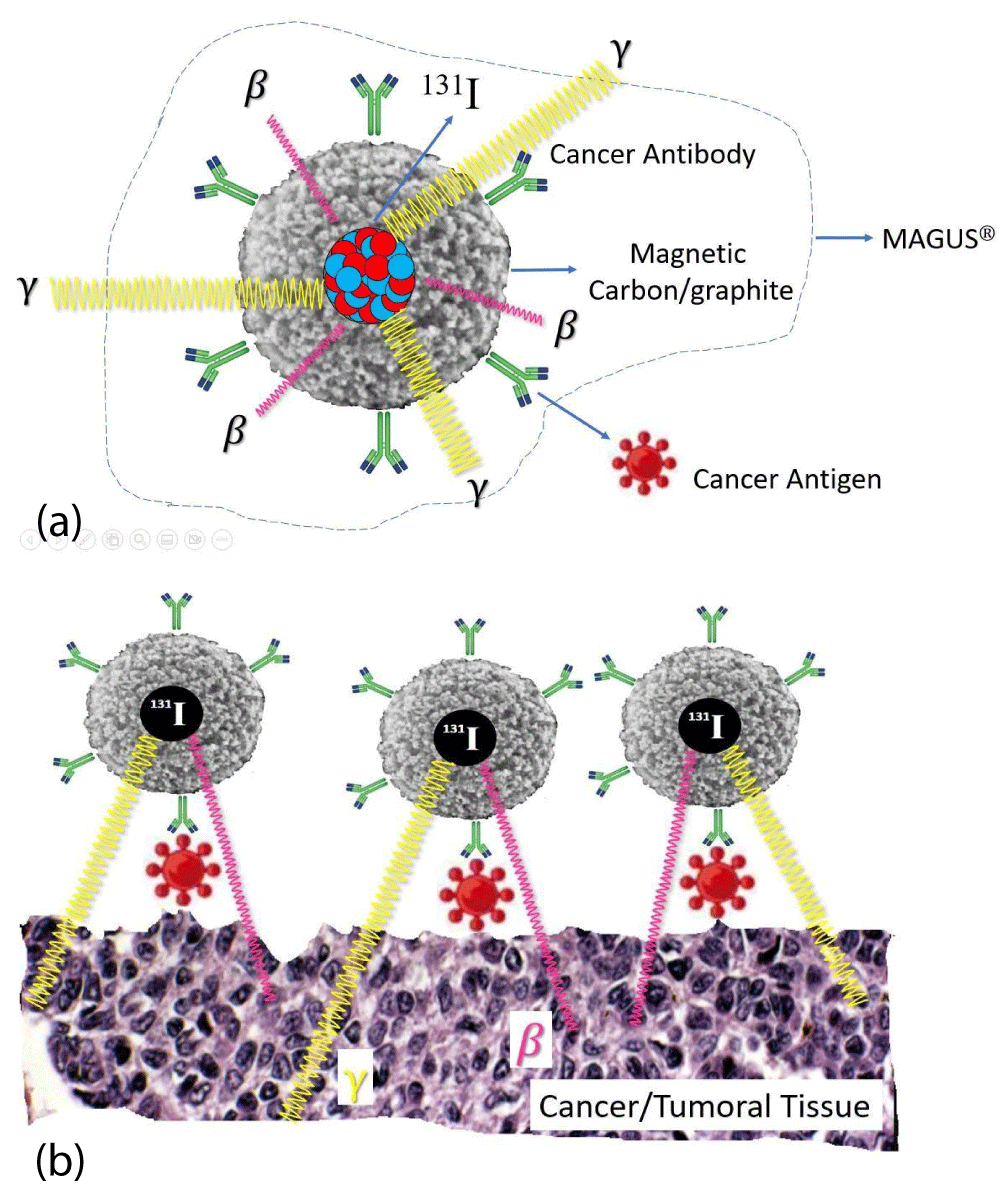
Besides the work carrying IBU described previously, we have also verified the concept and well-functioning of this complex carrier system by using the nanostructured biocompatible magnetic carbon/graphite functionalized with different cancer antibodies focusing on the antigen-antibody interaction besides other molecules and materials. These targeting techniques include functionalizing the magnetic carbon/graphite with radioactive nanoparticles like Technetium-99m, Indium-11, and Iodine-131. These radioactive nanoparticles can be produced by either synthesizing the nanoparticles directly from the radioactive materials or by irradiating non-radioactive particles with neutrons or accelerated ions [13]. Following this principle, at the present time, we are functionalizing the nanostructured biocompatible magnetic carbon/graphite with both Iodone-131 radioactive particles and the corresponding cancer antibody for targeting cancer cells (Figure 1). This isotope decays with a physical half-life of 8 days to stable Xe-131. It releases radiation during the decay process by emitting beta particles and gamma. The beta particles travel about 2 mm in tissue, thereby ensuring local treatment of the cancer tumor by causing mutation and death in cells that it penetrates. For this reason, high doses of the isotope are sometimes less dangerous than low doses since they tend to kill normal tissues that would otherwise become cancerous because of the radiation. Thus, Iodine-131 is increasingly less employed in small doses in medical use but increasingly is used only in large and maximal treatment doses, as a way of killing targeted cancer tissues. Iodine-131 is given for therapeutic use since about 10% of its energy and radiation dose is via gamma radiation while the other 90% is the beta radiation mentioned before.
Figure 1: (a) Sketch of MAGUS®, the nanostructured drug delivery system consisting of magnetic carbon/graphite functionalized with a radioactive particle of Iodine-131 for cancer irradiation treatment and the corresponding antigen-antibody interaction; (b) Interactions of those particles with cancer/tumoral tissue through antigen-antibody driven force; both figures show the radiation from Iodine-131 (beta and gamma).
Developing MAGUS® associated with boron neutron capture therapy (BNCT)
Another promising application we are at present working on is based on MAGUS® associated with Boron Neutron Capture Therapy (BNCT). This technique uses neutrons as the external source and is frequently used to treat specific tumors that are radio resistant or very difficult to kill using conventional radiation therapy [14].
It can be employed as a standalone radiation therapy or in combination with conventional radiotherapy methods. Some examples where it has proven to be very powerful and effective are in treating salivary gland tumors and certain forms of cancer, such as adenoid cystic carcinoma, inoperable/recurrent salivary gland malignancies resistant to standard low-LET radiotherapies and glioblastoma (high-grade glioma, GBM), a prevalent and aggressive brain tumor [15].
The BNCT uses Boron-containing drugs to deliver a natural isotope of the Boron-10 to tumors and while it is confined to tumors, as radionuclides tend to accumulate at the sites of tissue damage, a subsequent bombardment with neutrons provides an isotope of Lithium-7 and an alpha particle with a short range of action [13]. It means that the alpha particle deploys an amount of energy that is delivered in a high linear energy transfer (LET) due to its nature. In that case, their high energy will be delivered along their very brief pathway (<10 μm) conveying about 150 keV/μm. In other words, the dose is deposited inside a pathway that is the size of the diameter of a single cell [16].
Neutrons’ biological impact on cells is greater than other types of radiation. Since surprisingly they do not damage equally all cells, there are cases in which they can be more damaging to cancerous cells than to healthy cells surrounding cancer. Therefore, for the same amount of radiation, a lethal dose can be delivered to the cancer cells, while a sub-lethal dose is delivered to the healthy tissue reducing the chances of cell damage or death. Used thoroughly, this different impact can be an advantage in certain treatments. In general, neutron therapy shows high efficiency in the treatment of recurrent voluminous tumors of complex localization [16]. The approach we are working on for the BNCT application is based on functionalizing the nanostructured biocompatible magnetic carbon/graphite with Boron-10 (instead of Iodine-131) with the antibodies mentioned before. Then, we apply an external magnetic field to redirect the Boron-10 and employ the fast neutron dose more efficiently at the tumor, making it necessary for a lower dose to accomplish the same results. This is especially important for BNCT because fast neutron therapy is limited by high toxicity. And that is why we are providing once again to the system a double way to exclusively reach the target and not the healthy cells around increasing its efficiency and performance.
It is important to highlight that, by using both the interaction antigen-antibody and the guidance through an external magnetic field, we are affording our drug delivery system a double way to reach and act only the target, i.e., cancer and not the healthy cells around. Moreover, the target- specificity achieved by our delivery system MAGUS® comes from years of research of our group and represents a pioneering and effective way to treat cancer.
In conclusion, the nanostructured magnetic carbon/graphite we synthesize for the first time in 2005 by following an unprecedent simple chemical route, appears as a very promising way to achieve countless valuable goals mainly in medicine. It has shown itself to be superior in many aspects to the available solutions for some aggressive and prevalent types of tumors or even for recurrent voluminous tumors of complex localization due to its combined physical properties coupled with biological and physical functionalization. This has a special significance and relevance in nuclear science by considering functionalizing the nanostructured magnetic carbon/graphite with radioactive nanoparticles of Iodine-131 or Boron-10 following the BNCT technique to fight cancer with, most probably, no side effects. This is something that conventional drugs and other treatments do not have at all.
We are thankful to all our colleagues, partners, collaborators, students, and research agencies who contributed to this work for almost two decades. We also thank Brazilian funding agencies FAPESP, CAPES and FINEP for financial support.
- Galdino JF. The importance of integrating the military innovation system and the national innovation system; in: Collection of opinion articles on strategic studies in defense and security. ISBN 978-65-87080-44-4; JC Sanches, FM Araujo-Moreira. 2023; 155.
- GPT-4 Heralds An Enormous Productivity Boost, And A Wrenching Transformation Of Work (forbes.com) (consulted in March, 2023.
- Cancer statistics. 2022 - PubMed (nih.gov) (consulted in March 2023).
- Cancer (who.int) (consulted in March 2023).
- Mombrú AW, Pardo H, Faccio R, De Lima OF, Leite ER, Zanelatto G, Lanfredi AJC, Cardoso CA, Araújo-Moreira FM. Multilevel ferromagnetic behavior of room- temperature bulk magnetic graphite; Phys. Rev. B (Rapid Comm.). 2005; 71:100404(R).
- Pardo H, Faccio R, Araújo-Moreira FM, De Lima OF, Mombrú AW. Synthesis and characterization of stable room temperature bulk ferromagnetic graphite; Carbon. 2006; 44:565–569.
- Souza NS, Sergeenkov S, Speglich C, Rivera VAG, Cardoso CA, Pardo H, Mombrú AW, Rodrigues AD, De Lima OF, Araújo-Moreira FM. Synthesis, characterization, and magnetic properties of room-temperature nanofluid ferromagnetic graphite; Appl. Phys. Lett. 2009; 95:23; 233120.
- Freitas JC, Scopel WL, Paz WS, Bernardes LV, Cunha-Filho FE, Speglich C, Araújo-Moreira FM, Pelc D, Cvitanić T, Požek M. Determination of the hyperfine magnetic field in magnetic carbon-based materials: DFT calculations and NMR experiments. Sci Rep. 2015 Oct 5;5:14761. doi: 10.1038/srep14761. PMID: 26434597; PMCID: PMC4593005.
- Ribeiro LN, Alcântara AC, Darder M, Aranda P, Herrmann PS Jr, Araújo-Moreira FM, García-Hernández M, Ruiz-Hitzky E. Bionanocomposites containing magnetic graphite as potential systems for drug delivery. Int J Pharm. 2014 Dec 30;477(1-2):553-63. doi: 10.1016/j.ijpharm.2014.10.033. Epub 2014 Oct 16. PMID: 25455784.
- Souza NS, Sergeenkov S, Rodrigues AD, Cardoso CA, Pardo H, Faccio R, Mombrú AW, Galzerani JC, De Lima OF, Araujo-Moreira FM. Stability issues and structure-sensitive magnetic properties of nanofluid ferromagnetic graphite; J. of Nanofluids. 2012; 1:143–147.
- Souza NS, Rodrigues AD, Cardoso CA, Pardo H, Faccio R, Mombru, Galzerani JC, De Lima OF, Sergeenkov S, Araujo-Moreira FM. Physical properties of nanofluid suspension of ferromagnetic graphite with high Zeta potential; Phys. Lett. A. 2012; 376:4; 544-546.
- Araujo-Moreira FM, Serrano NFG. Stable Room-Temperature Magnetic Carbon Graphite: From Discovery to Bionanotechnological Applications; Research and Development in Material Science. 2022; 17: 2.
- Abbas K, Simonelli F, Holzwarth U, Gibson P. Overview on the production of radioactive nanoparticles for bioscience applications at the JRC Cyclotron – European Commission; Journal of Labelled Compounds and Radiopharmaceuticals. 2009; 52: S231–S255.
- Malouff TD, Seneviratne DS, Ebner DK, Stross WC, Waddle MR, Trifiletti DM, Krishnan S. Boron Neutron Capture Therapy: A Review of Clinical Applications. Front Oncol. 2021 Feb 26;11:601820. doi: 10.3389/fonc.2021.601820. PMID: 33718149; PMCID: PMC7952987.
- Kiseleva V, Gordon K, Vishnyakova P, Gantsova E, Elchaninov A, Fatkhudinov T. Particle Therapy: Clinical Applications and Biological Effects. Life (Basel). 2022 Dec 9;12(12):2071. doi: 10.3390/life12122071. PMID: 36556436; PMCID: PMC9785772.
- Matsumoto Y, Fukumitsu N, Ishikawa H, Nakai K, Sakurai H. A Critical Review of Radiation Therapy: From Particle Beam Therapy (Proton, Carbon, and BNCT) to Beyond. J Pers Med. 2021 Aug 23;11(8):825. doi: 10.3390/jpm11080825. PMID: 34442469; PMCID: PMC8399040.