Research Article
Neutralizing scFv Antibodies against Infectious Bursal Disease Virus Isolated From a Nlpa-Based Bacterial Display Library

Tianhe Li1*, Bing Zhou2*, Tingqiao Yu3, Ning Li4, Xiaochen Guo2, Tianyuan Zhang2, Jingzhuang Zhao2, Liming Xu2, Siming Li2, Lei Ma2, Tingting Li2, Liangjun Ding2, Mingzhe Sun2, Deshan Li2# and Jiechao Yin2#
1Beijing Obstetrics and Gynecology Hospital, Capital Medical University Beijing Maternal and Child Health Care Hospital, China
2Biopharmaceutical Lab, College of Life Science, Northeast Agricultural University, China
3College of Biological Sciences and Biotechnology, Beijing Forestry University, China
4Patent Examination Cooperation Center of the Patent Office, SIPO, Beijing, China
#Authors contributed equally to this study
*Address for Correspondence: Tianhe Li, Beijing Obstetrics and Gynecology Hospital, Capital Medical University Beijing Maternal and Child Health Care Hospital, China, Tel: 0451-55190645; Email: [email protected]
Bing Zhou, Biopharmaceutical Lab, College of Life Science, Northeast Agricultural University, China, Tel: 0451-55190645
Dates: Submitted: 20 January 2017; Approved: 15 February 2017; Published: 21 February 2017
How to cite this article: Li T, Zhou B, Yu T, Li N, Guo X, et al. Neutralizing scFv Antibodies against Infectious Bursal Disease Virus Isolated From a Nlpa-Based Bacterial Display Library. Ann Adv Chem. 2017; 1: 001-011. DOI: 10.29328/journal.aac.1001001
Copyright License: © 2017 Li T, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: IBDV; Prokaryotic expression; Bacterial display; Antibody library; Neutralization scFv antibody
ABSTRACT
Infectious bursal disease (IBD) considered as one of the major viral diseases threatening the poultry industry worldwide. The causative agent of the IBD is Infectious bursal disease virus (IBDV) which replicates in developing B lymphocytes in the bursa of Fabricius leading to its destruction and bursal inflammation. In this study, we investigated a technology to produce therapeutic recombinant antibodies against IBDV in bacteria by constructing a bacterial displayed recombinant scFv library from immunized chickens, followed by screening the scFv library by fluorescence activated cell sorting (FACS) with FITC-labeled VP2. Twelve VP2-binding scFv clones with unique sequences were obtained, with overall amino acid homology of 81.53%. The complementarity determining region (CDR) 3 in the heavy chain displayed the lowest homology, while the amino acid sequences in framework regions and CDR2 of both chains and CDR1 of the heavy chain are relatively conserved. Twelve VP2-binding scFv clones were expressed in E.coli and purified through denaturation and denaturation of inclusion bodies. Our ELISA results showed that all scFvs exhibited binding ability and specificity to VP2 and various IBDV strains. In addition, two scFvs showed significant neutralizing activity to IBDV (B-87 strain) as these scFvs inhibited cytopathic effect of chicken embryo fibroblast (DF1) caused by IBDV. In conclusion, our study provides a lead candidate for further development of therapeutic antibodies for IBDV infection.
INTRODUCTION
Infectious bursal disease (IBD) was initially identified in chicken in 1957, IBD is being considered as one of the major diseases threatening the poultry industry worldwide [1]. IBD is caused by the infectious bursal disease virus (IBDV), a double stranded RNA virus belonging to the family Birnaviridae [2]. IBD is an acute and highly contagious among chicken, and characterized as highly contagious immunosuppressive in chickens by rapid replication of IBD virus within the bursa of Fabricius and depleting B cell populations [3], and increased susceptibility to other diseases such as bacterial infection or viral infections [4]. Recent years, due to prevalence of the very virulent IBDV, prevention and treatment of IBDV become more important [5].Vaccination is an important mean for prevention of IBDV prevalence [6]. Live vaccines show different degrees of attenuation; many of them may cause bursal atrophy and thus immunosuppression with poor immune response to vaccination against other pathogens and an increase in vulnerability to various types of infections [6]. Passive hyper immune therapy (PHT) is an alternative for standard vaccination, and is characterize by the advantage of immediate acquired the immunity once injection, and passive immunization with antibodies are widely used to prevent or treat infections like measles, hepatitis A, tetanus, varicella, and vaccinia [7,8]. Hyper immune serum and egg yolk antibodies may have good effects in the early onset of IBD, but are restricted by high cost or poor safety. Genetically engineered antibodies represent a viable alternative for prevention and treatment of IBDV infection [9,10]. The present study is isolate neutralizing antibodies against IBD using NLPA-based bacterial display technology from combinatorial scFv library. Twelve scFv clones were identified as possessing binding ability and specificity to VP2 and different IBDV strains. Importantly, two of them named s-29 and s-40 possess neutralizing capacity for IBDV, providing promising candidates for further development of therapeutic antibodies for prevention and treatment of IBDV infection.
MATERIALS AND METHODS
Materials
Strains, Vectors and Reagents: DF1cells, pET-27b vector, E.coli DH5α, Rosetta (DE3) were lab stocks, cloning vector containing (G4S)3 gene, B-display vector containing the sequence of NlpA leader +6aa were constructed by our laboratory. VP2 protein was expressed and purified by the lab. Anti-IBDV egg yolk antibody was extracted by lab. IBDV vaccine strain B-87 was purchased from Harbin Pharmaceutical Group. DNA marker and pMD18 T-simple-vector were purchased from TaKaRa. HRP-rabbit anti-chicken antibody was purchased from eBioscienc. Protein marker was purchased from Ferments. The primers were synthesized by Invitrogen (Table 1).
| Table 1: The primers for cloning scFv. The sequences of chicken scFv heavy chain and light chain were checked in NCBI. Primers of heavy chain (HF, HR), light chain (LF, LR) and restriction enzyme sites were designed by Primer5. All primers were synthesized by Invitrogen. The restriction sites were underlined. | |
| Primers (restriction sites) | Sequences |
| HF(HindⅢ) | CTAAAGCTTGCCGTGACGTTGGACGAG |
| HR (NheI) | CGCGCTAGCGGAGGAGACGATGACTTCGGTCC |
| LF ( BamHI) | CGCGGATCCGCGCTGACTCAGCCGTCCTCGGTGTC |
| LR (XhoI) | CCGCTCGAGTTAACCTAGGACGGTCAGGG |
Methods
Construction of the scFv Bacterial Displaying Library against Vp2
Immunization of Chicken: Three specific pathogen-free (SPF) chickens were immunized by intra-ocular administration of IBDV vaccine strain B-87 in the dose of 107pfu, the chickens were boosted one week later by intra-muscular injection with 0.5ml of formalin-inactivated preparation of B-87 emulsified with an equal volume of Freund’s incomplete adjuvant. Four weeks after the secondary vaccination, the titer of immune serum was determined by ELISA, chickens were euthanized and spleens were collected for extraction of RNA by Trizol.
cdna Synthesis from Spleen Total Rna of The Immunized Chicken
Splenocytes were isolated from the immunized chicken for RNA extraction, and total RNA was extractd using Trizol. cDNA was synthesized from total RNA sample using Superscript II (Invitrogen) and random hexamer oligonucleotide pimers (2 μg).
Construction of scFv library
Primers for scFv designed based on the variable region gene sequence in GenBank of chicken Light chain and Heavy chain. VH primers contained the restriction sites of HindⅢ/NheI and VL primers contained the restriction sites of BamHI/XhoI.
The cloning vector pYDx was derived from pYD-1 [11], by creating restriction enzyme sites of NheI and BamHI, the resulting HindⅢ/NheI for cloning VH and BamHI/XhoI for cloning VL. The display vector B-display containing NlPA leader+6aa for inner membrane anchoring and compatible RE sites with the cloning vector was constructed from expression vector pET27b.
The VH and VL gene pool against IBDV were amplified by PCR from the cDNA. The vector first cleaved with HindⅢ and NheI and ligated with the VH fragment and then cleaved with BamHI and XhoI and ligated with the the VL fragment after gel purification of the ligation products. The recovery VH and VL fragments were cloned up and downstream of scFv-peptide-linker (G4S)3 gene of pYDx-vector. The library was termed as pYDx-scFv library. The VH-linker-VL fragments were then digested by Hind/XhoI and cloned into B-display vector. The resulting library was termed B-display-scFv library. Library diversity was determined by DNA sequencing.
Screening and detection of anti-Vp2 scFv bacterial displaying library
All colonies about 107 cells from the B-display-scFv library-transformed DH5ɑ were collected and cultured in LB media, When the OD600 reached 0.3~0.4, isopropylβ-D-thiogalactoside (IPTG) was added into the medium at the final concentration of 0.25 mmol/L and incubated at 37℃ for 4 h. One milliliter bacterial cells was collected and washed with PBS for 2 times, the cells were resuspended in 350 ml of ice-cold solution of 0.75 mol/L sucrose/0.1 mol/L Tris-HCl (pH 8.0) and added 35 μl lysozyme (10 mg/mL) to cells. The cells were then treated with 700 μl of ice-cold EDTA (1 mmol) and 50 μL of MgCl2 (0.5 mol). The cells pellet was resuspended in 100 μl PBS gently and incubated with 4 μl FITC labeled VP2 (2 mg/mL) protein and 1 μl bovine serum albumin (BSA 1%) at 4℃ for 1h. Then the cells were washed four times and resuspended in 500 μl PBS for FACS analysis. The scFv display library was screened with FACS after several rounds of screening, 30 single colonies were picked at random and VP2-binding scFv clones were confirmed by the FACS and subjected to DNA sequencing.
Construction of the recombinant plasmids for expression of anti-Vp2 scFv genes
Anti-VP2 scFv genes were amplified by PCR from plasmids containing the VP2-binding clones i.e. B-display-s-1; B-display-s-12; B-display-s-17; B-display-s-19; B-display-s-25; B-display-s-29; B-display-s-30; B-display-s-32; B-display-s-38; B-display-s-40; B-display-s-50 and B-display-s-220 sub cloned into the pET-27b vector. Recombinant plasmids were transformed into E.coli Rosetta for expression.
The expression and purification of recombinant proteins
Single colonies of E.coli Rosetta (containing recombinant plasmids of pET-scFv and pET-VP2) were grown in LB media containing kanamycin (50 μg/mL), when the OD600 reached 0.3~0.4, scFv expression was induced by IPTG into the medium to the final concentration of 0.5 mmol/L and incubated at 37℃ for 4 h. One milliliter of bacteria was collected and ultrasonicated at 150 w for 1 min, the supernatant and pellet (resuspended in PBS) were separated and SDS-PAGE (sodium dodecyl sulfate- polyacrylamide gel electrophoresis) was used to analysis expression of recombinant protein. Inclusion bodies were refolded through gradual reduction of urea. The inclusion body was harvested by centrifugation at 10,000 g for 30 min, and resuspended in solution buffer (2 mol/L Urea) after 2 washes. The pellet was then dissolved in denaturation solution (8 mol/L Urea) and stored overnight at 4℃. Ten volumes of renaturing solution (2 mol/L Urea) were added to the denaturation solution slowly and stored at 4℃ for 24 h. The supernatant was harvested by centrifugation and dialyzed in PBS overnight for desalination. SDS-PAGE and HPLC were used to analyze the purity of the recombinant proteins after dialysis in PBS.
Elisa
96-well microliter plates were coated with the purified recombinant proteins scFvs at different concentrations in NaHCO3/NaCO3 buffer at pH 8.7 for overnight at 4℃. The triplicate samples were washed twice with PBS-Tween (0.5%), and 5% skim milk in PBS was used to blocking the remaining non-specific binding sites at 37℃ for 2 h. After washing, each wells were incubated with VP2 protein (40 μg/mL) or others IBDV strains, at 37℃ for 1 h, followed by secondary antibody (diluted by 200-fold) and HRP-rabbit anti-chicken antibody (diluted by 7500-fold). The assay was developed using TMB solution the development of color product was terminated by 50 μl of 0.1mol H2SO4. The absorbance of each well was measured with an ELISA reader at wave length of 450 nm.
Titration of IBDV
The IBDV virus solution was derived by passaging of IBDV vaccinate (B-87 strain, 100 μl) in chicken embryos. IBDV was cultured in chicken embryos. Following the death of the chicken embryo was dead two to three days post inoculation, the chicken embryo allantois solution was harvest. Two rounds of proliferations were performed in chicken embryo as above.
The chicken embryo fibroblasts cells (DF1 cell) were maintained in DMEM with 10% FBS with MDEM, the IBDV was diluted with DMEM. The exponentially DF1 cells were seeded into 96-well plates (100 μl in each well) and the monolayer DF1 cells were treated with Log2 dilution of IBDV (100 μl in each well), and incubated at 37℃ in the presence of 5% CO2. Cells were examined visually for cytopathic effect about 4-6 days. Control cells were treated in the same way but 100 μl DMEM was used to instead of IBDV. Samples were measured in 8 replicates and each experiment was repeated at least twice. The 50% tissue culture infective dose (TCID50) of virus was calculated by Reed-Muench Method.
The neutralizing activity of scFvs antibody to IBDV
Log2 dilutions of scFvs (100 μl) from 300ng/ μl to 0.586ng/ μl were incubated with 100TCID50 of IBDV (B-87, 100 μl) for 1 h at 37℃. ScFvs and virus mixture were then added to a freshly prepared of DF1 monolayer in 96-well tissue culture plates and incubated at 37℃ in the presence of 5% CO2. One control cells were treated with IBDV (100TCID50, 100 μl), another were treated with DMEM (100 μl). Cells were examined visually for 4-6 days by microscope. All samples were measured in 8 replicates.
RESULTS
Construction of the bacterial displaying scFv library from the spleen of IBDV-immunized chicken
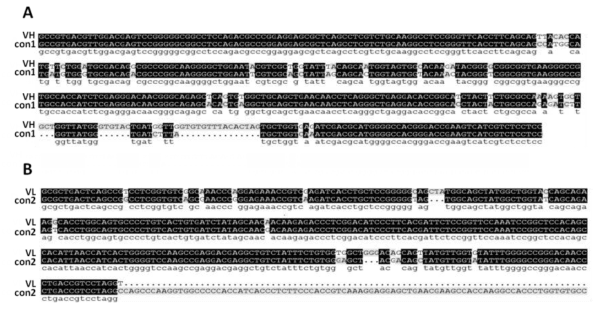
For the purpose of scFv library, the cDNA for cloning VH and VL was derived from the spleen of IBDV-immunized chicken for obtaining the anti-IBDV antibody genes. The length of VH is about 400 bp, the length of VL is about 320 bp. The PCR product was sequencing and results show the VH and VL has a high homology with control (Figure 1A,B), confirming that the selected clones were chicken scFv genes. The capacity was above 1.3×108 for construction of B-display-scFv library that provide a foundation of library screening.
Screening of the scFv library against VP2 by FACS
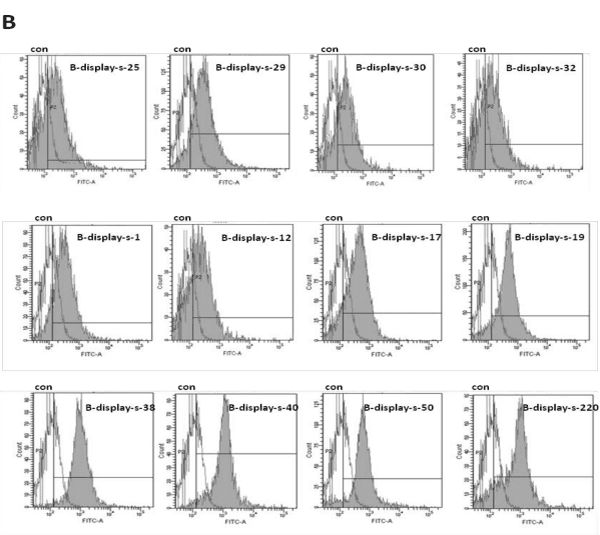
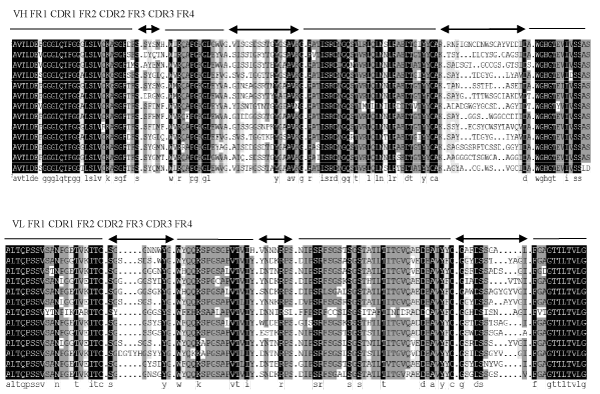
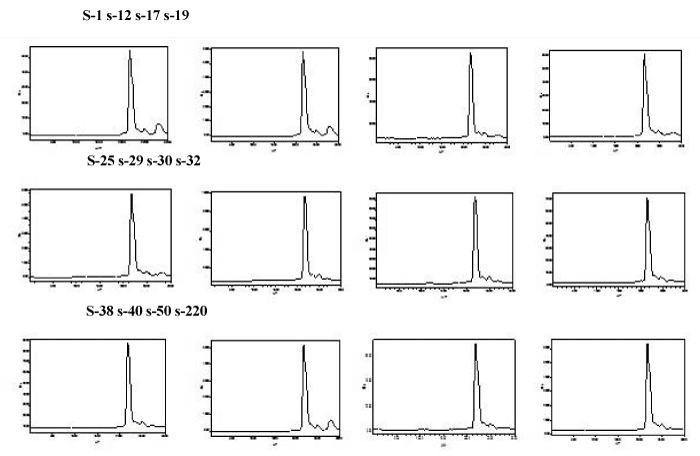
The bacterial display library was subjected to three rounds of screening by FACS. Results shown, compare with untreated cell, positive scFv antibodies increase with the increase of screening times. When the peak of control and sample have separated and VP2-binding population reached 50%, 30 single colonies were randomly picked and confirmed by FACS analysis. Twelve clones, which bound to VP2 antigen, were selected and named as B-display-s (Figure 2). The DNA sequence of VP2-binding scFvs was determined and the amino acid sequence deduced. The rest of the information “FR1-4 and CDR1-3 were designated according to Sapats et al., Analysis of the amino acid sequence reveals that all twelve scFvs possess unique VH and VL sequences. The homology among scFvs is 81.53%, and is up to 95% in frame regions of scFvs. The homology of the heavy chain CDR is lower than that of light chain CDR, and CDR3 of the heavy chain has the lowest homology. The homology of the heavy chain CDR1, CDR2 and CDR3 is 55.67%, 69.61%, 35.32%, respectively. The homology of light chain CDR1, CDR2 and CDR3 is 51.28%, 69.05%, 52.38%, respectively. There is no difference in the length of amino acids among FRs, for example, the length of amino acids the heavy chain FR1-FR4 is 30, 14, 32 and 13, respectively. The length of amino acids of the light chain FR1-FR4 is 20, 16, 32 and 11, respectively. The length of amino acids of heavy chain CDR1 and CDR2 is 5, 17, and the CDR3 is 20-14. The length of amino acids of light chain CDR2 is 7, and CDR1, CDR3 is 18-13, 14-29, respectively (Figure 3).
Figure 2: Twelve VP2-binding scFv clones obtained from the bacterial display library. The solid peaks indicate scFv-transformed cells which were incubated with 4 μl FITC-labeled VP2 (2 mg/mL) and detected by FACS. The hollow peaks indicate untreated cells which were used as negative controls, the twelve VP2-binding clones are named as B-display-s-1; B-display-s-12; B-display-s-17; B-display-s-19; B-display-s-25; B-display-s-29; B-display-s-30; B-display-s-32; B-display-s-38; B-display-s-40; B-display-s-50 and B-display-s-220.
Figure 3: Alignment of the amino acid sequences of the heavy and light chain genes of scFvs clones with consensus chicken scFv (con). VP2-binding scFvs were confirmed by DNA sequencing, the FR1-4 and CDR1-3 were divided according to S. I. Sapats et al. Complementarity determining regions (CDR1-3) are shown on top of the sequence by arrows and Frame region (FR1-4) are shown by lines. The Gen Bank number of consensus sequence is AAO15864.1.
The expression and purification of anti-VP2 scFv
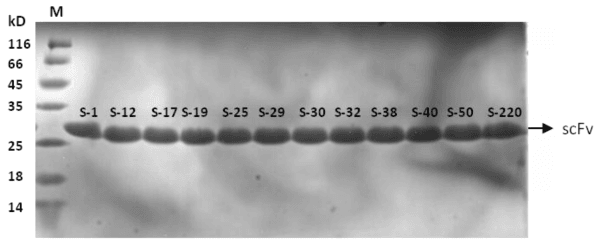
The scFv genes from B-display-scFv plasmids were sub cloned into the expression vector of pET27b to construct the recombinant plasmids pET-scFvs for expression. The scFvs were expressed as an inclusion bodies. Therefore after denaturing and refolding, the purified proteins of the twelve scFvs were obtained. SDS-PAGE analysis showed that the purified scFv proteins were approximately 28kD (Figure 4). The recombinant scFvs were named as s-1, s-12, s-17, s-19, s-25, s-29, s-30, s-32, s-38, s-40, s-50 and s-220. The scFvs concentrations are approximately 0.5mg/ml. HPLC results showed the purity of scFvs was close to 85% (Figure 5).
Figure 4: SDS-PAGE analysis of the purified anti-hIL-1β scFvs. Twelve VP2-binding scFvs were expressed by E.coli and purified by denature and renature the inclusion body. Lane1: Protein marker, Lane2-13: s-1, s-12, s-17, s-19, s-25, s-29, s-30, s-32, s-38, s-40, s-50, s-220.
Figure 5: HPLC analyse the purity of scFvs. The purity of scFvs were up to 85%. Abscissa denotes the appear time of target protein, ordinate denotes ultraviolet absorption value at 280nm.
Binding ability of the twelve scFvs with VP2 and different IBDV Strains
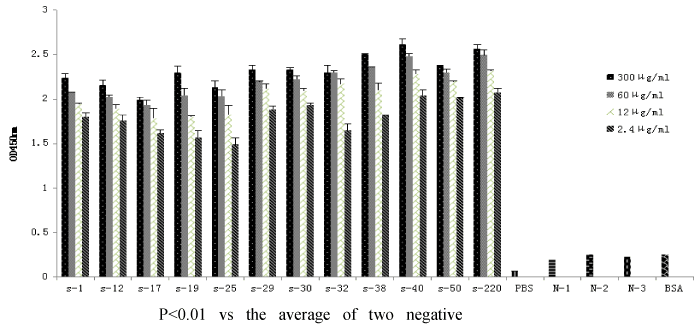
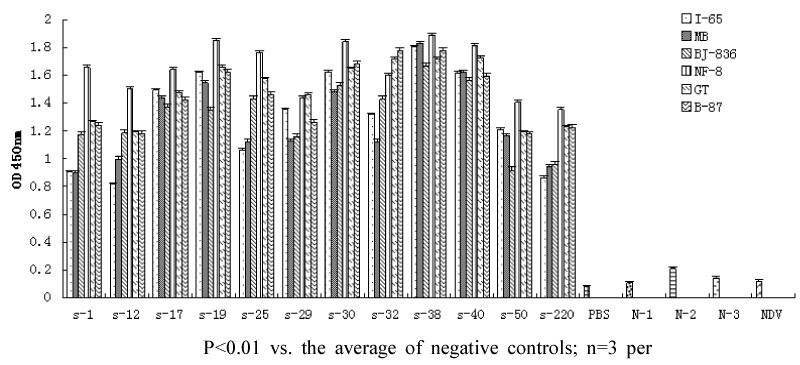
Binding ability of the anti-VP2 scFvs to VP2 and different IBDV strains was determined by sandwich ELISA assay using 96-well plates coated with purified recombinant scFv. The ELISA results for VP2 binding indicated that the value of OD450 nm increased with higher amounts of scFvs, whereas the values of negative controls were negligible (Figure 6). All scFvs showed binding ability to the six different IBDV strains tested and did not bind NDV (Figure 7).
Figure 6: ELISA analysis of the binding ability of anti-VP2 scFvs to VP2. The plates were coated with different concentrations (300 μg/mL,60 μg/mL,12 μg/mL,2.4 μg/mL) of scFvs, followed by incubation with VP2, chicken egg yolk antibody and secondary antibody. control1 (without VP2 or IBDV strains), control2 (without egg yolk antibody), control3 (without VP2 and egg yolk antibody), control4 (with BSA to replace VP2, or with Newcastle disease virus (NDV) to replace IBDV), and PBS was as background. S denotes scFv, N denotes control. BSA denotes VP2 was replaced by BSA.
Figure 7: The binding ability of anti-VP2 scFvs to different IBDV strains by Elisa. The plates were coated with 300μg/mL scFvs, followed by incubation with different IBDV strains (include I-65, MB, BJ-836, NF-8, GT, B-87), chicken egg yolk antibody and secondary antibody. control1 (without VP2 or IBDV strains), control2 (without egg yolk antibody), control3 (without VP2 and egg yolk antibody), control4 (with BSA to replace VP2, or with Newcastle disease virus (NDV) to replace IBDV), and PBS was as background. S denotes scFv, N denotes control. NDV denotes IBDV was replaced by NDV.
Neutralization of IBDV Infectivity by scFvs in vitro
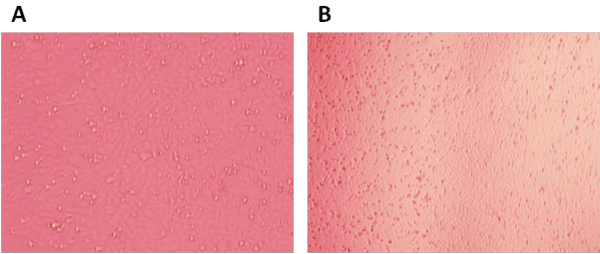
Neutralization experiment performed to determine the ability of scFvs to neutralize the tissue culture adapted IBDV. Compared with untreated cell (Figure 8A), the DF1 cell death when treated with IBDV (Figure 8B). The CPE was observed after treated scFv antibodies and IBDV for 4-6 days. Result show that two of the scFvs were able to neutralize 100TCID50 of IBDV, one scFv antibody (s-40) can inhibit the effect of IBDV at a low protein concentration (2.344 ng/ μl), it showed high neutralizing ability. One scFvs (s-29) show neutralization ability when it concentration above 150 ng/ μl, whilst 10 scFvs (s-1, 12, 17, 19, 25, 30, 32, 38, 50, 220) can’t neutralized IBDV even at a high antibody concentration, their no neutralizing properties (Table 2).
Figure 8: The CPE of DF1 cell. The IBDV was added into DF1 cell, and CPE was observed by microscope. Untreated cell (A), IBDV treated cell (B).
| Table 2: Titration of scFvs antibody against IBDV (B87 strain). After treated DF1cells with different concentration of scFv antibody and 100TCID50 of IBDV for 4-6 days. The CPE was observed by microscope, the cytopatic effect indicate the proportion of cell death. | ||||||||||||
| Concentration(ng/µl) | s-1 | s-12 | s-17 | s-19 | s-25 | s-29 | s-30 | s-32 | s-38 | s-40 | s-50 | s-220 |
| 300 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 0 | 100 | 100 |
| 150 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 0 | 100 | 100 |
| 75 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| 37.5 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| 18.75 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| 9.375 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| 4.688 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| 2.344 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 100 | 100 |
| 1.172 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 0.586 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
DISCUSSION
Passive immunization by administration of specific antibodies has been widely used in animal and presents an attractive approach to prevention and treatment of IBD. Hyper-immune serum is seldom produced in large-scale due to the potential risks to transmit infectious diseases, difficulty in obtaining large quantities of blood and concerns about animal welfare. In 1962 it was found that immunoglobulin concentration in the yolk was equal to or greater than that found in hen serum and Muhammad et al. (2001), demonstrated that yolk antibodies from hyper immunized hens could be used to control IBD in commercial laying hens. For this purpose, the yolk antibodies have been manufactured in some countries. However, there are various shortcomings for using yolk antibodies in prevention and treatment of IBDV infection. First, because the antibodies are derived from the natural host, there are potential risks to transmit the infectious diseases. Second, the concentration of the specific antibody against IBDV may be low (2%-10%). Third, the production cost for the high quality IgY antibodies are relatively high. Traditional monoclonal antibodies from mice can overcome these shortcomings, but they cannot be used for prevention and treatment of IBDV infection due to strong immunologic rejection12. To date, the best option to overcome these challenges might be genetically engineered antibodies from chickens, which offers a number of advantages. First, there is no concern for contamination of infectious pathogens during the production process. Second, specific and monoclonal antibodies with high affinity are obtained by gene manipulation technology. Third, the manufacturing process and quality control procedure can be easily established. Therapeutic antibodies for human diseases like cancers, autoimmune diseases and virus infections are dominantly studied; the humanized monoclonal antibody (Palivizumab) against respiratory syncytial virus had been proved decade ago [13]. However, genetically engineered therapeutic antibodies for animal diseases have not been reported. The aim of the current study was to isolate chicken monoclonal antibodies with neutralizing capacity against IBDV for prevention and treatment of IBD, to overcome the problems with the egg yolk antibodies and establish the technology platform for development of therapeutic antibodies for other animal diseases [14].
In this study, we described twelve recombinant scFv antibodies isolated from the library derived from the spleen of the immunized chicken with IBDV. Our ELISA results clearly indicate that our scFv antibodies possess strong binding ability and specificity to VP2 and various IBDV vaccine strains I-65, MB, BJ-836, NF-8, GT and B-87. According to the ELISA titers the binding affinity to different IBDV vaccine strains varied, suggesting that the amino acid variations in the antigenic epitopes may exist. It is known that IBDV can cause cytopathic effect (CPE) in DF1 cells. The scFv can be considered as a neutralizing antibody if it can block the IBDV-induced CPE in DF1 cells. One of scFvs , namely s-40, demonstrates a high neutralizing activity to IBDV-B-87, the lowest concentration to inhibit IBDV-B-87 (100TCID50) is 2.344ng/ μl, and one of scFvs, s-29, demonstrates a lower level of neutralizing activity, the lowest concentration to inhibit IBDV-B-87 (100TCID50) is 150ng/ μl. Because these scFvs can neutralize the IBDV-B-87 infection in vitro, they have potential to be developed as therapeutic antibodies and to replace the hyper immune egg yolk antibodies for prevention and treatment of IBDV infection in vivo. Hence, this study established technical platform for development of genetically engineered and species-originated monoclonal antibodies for prevention and treatment of other viral diseases.
Currently, the major tools for isolating antibodies from large recombinant libraries are protein display technologies such as phage-display, which have become the important method for generating recombinant antibodies for research and clinic application [10,15]. The phage display technology has a high non-specificity [16]. Whereas, the NLPA-based bacterial display technology offers an efficient way to process library screening with FACS, which have the advantages of much large library size, and faster growth of E.coli to diminish the screening time and enables real-time visualization to identify the desire antibody clones [17]. In addition, our unpublished data indicated that all scFvs express at a similar level in the bacterial periplasm and fluorescent intensity shown in FACS analysis can reflect the binding affinity of the individual scFv, which is confirmed by ELISA assay. Therefore, the bacterial display in combination with FACS can be used for quantitative and real time selection of desirable antibody clones with different binding affinity. In this study, the size of library was about 1.3×108, and the positive clones can be enriched up to 50% after three round screening, and twelve anti-VP2 scFvs were isolated. Our results prove that the bacterial display technology is not only applied for antibody mutation but also for screening combinatorial scFv libraries.
Chickens possess only one functional immunoglobulin heavy chain variable region (VH) gene and one light chain variable region (VL) gene, gene conversion arises by the incorporation of pseudo V region genes resulting in the diversity of chicken antibodies [18]. So, all chicken antibodies V regions can be expected to have virtually identical amino acid sequence at both termini of the heavy chain and light chain [19]. As a result, the single set of PCR primers designed around the conserved regions of functional VH and VL genes enables to amplify the complete spectrum of rearranged variable fragments and clone highly diverse chicken immunoglobulin repertoires. Therefore, recombinant antibody libraries of high diversity are technically easier to generate from chickens than other mammalian species, due to the peculiar mechanism of immunoglobulin diversification in avian. In this study, we utilized the advantage of chicken antibody library in combination with the high through put bacterial technology to isolate the neutralizing antibodies for prevention and therapeutic purposes, this technology is not only used to isolate antibodies for chicken pathogens, but also for pathogens of other animals.
ScFv is a small molecule form of antibody, and can be used as a diagnostic or therapeutic agent [20]. Advantages of scFv antibodies are their solubility, rapid tissue penetration and recognition of hidden antigenic sites, to be easily constructed for Fab and full-length antibody as well as cost-effective production in micro-organisms [21]. In the present study, we described twelve recombinant scFv antibodies isolated from an anti-IBDV library derived from the spleen of the immunized chicken by the bacterial display system These scFv antibodies show binding ability and specificity to VP2 and different IBDV strains and two of them demonstrates a neutralizing activity, suggesting that these clones have potential for development of therapeutic antibodies.
ACKNOWLEDGEMENT
This work was supported by Heilongjiang Province Project of Applied Technology and Development (2013GC13C105) and the National Science Fund biologic science base improve program of research training and capacity (J1210069/J0124).
REFERENCES
- Greenall SA, Tyack SG, Johnson MA, Sapats SI. Antibody fragments, expressed by a fowl adenovirus vector, are able to neutralize infectious bursal disease virus. Avian pathol. 2010; 39: 339-348. Ref.: https://goo.gl/AGydXt
- Busnadiego I, Maestre AM, Rodriguez D, Rodriguez JF. The infectious bursal disease virus rna-binding vp3 polypeptide inhibits pkr-mediated apoptosis. PloS One. 2012; 7. Ref.: https://goo.gl/WN9yFn
- Smith J, Sadeyen JR, Butter C, Kaiser P, Burt DW. Analysis of the early immune response to infection by infectious bursal disease virus in chickens differing in their resistance to the disease. J Virol. 2015; 89: 2469-2482. Ref.: https://goo.gl/W4N32a
- Deb R, Dey S, Madhan Mohan C, Gaikwad S, Kamble N, et al. Development and evaluation of a salmonella typhimurium flagellin based chimeric DNA vaccine against infectious bursal disease of poultry. Res Vet Sci. 2015; 102: 7-14. Ref.: https://goo.gl/eDDjPl
- Guo X, Wang L, Cui D, Ruan W, Liu F, et al. Differential expression of the toll-like receptor pathway and related genes of chicken bursa after experimental infection with infectious bursa disease virus. Arch Virol. 2012; 157: 2189-2199. Ref.: https://goo.gl/gy0MVL
- Hornyak A, Lipinski KS, Bakonyi T, Forgach P, Horvath E, et al. Effective multiple oral administration of reverse genetics engineered infectious bursal disease virus in mice in the presence of neutralizing antibodies. J Gene Med. 2015; 17: 116-131. Ref.: https://goo.gl/5a3a02
- Mahgoub HA, Bailey M, Kaiser P. An overview of infectious bursal disease. Arch Virol. 2012; 157: 2047-2057. Ref.: https://goo.gl/Cn1ddg
- Su BS, Chiu HH, Lin CC, Shien JH, Yin HS, et al. Adjuvant activity of chicken interleukin-12 co-administered with infectious bursal disease virus recombinant vp2 antigen in chickens. Vet Immunol Immunopathol. 2011; 139: 167-175. Ref.: https://goo.gl/ZlLGqw
- Xu Y, Li X, Jin L, Zhen Y, Lu Y, et al. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: A review. Biotechnol Adv. 2011; 29: 860-868. Ref.: https://goo.gl/zEITFB
- Li K, Courtillon C, Guionie O, Allee C, Amelot M, et al. Genetic, antigenic and pathogenic characterization of four infectious bursal disease virus isolates from china suggests continued evolution of very virulent viruses. Infect Genet Evol. 2015; 30: 120-127. Ref.: https://goo.gl/J4KUoq
- T Li, L Xu, G Ren, B Zhou,Ye X, et al. A neutralization scfv antibody against il-1β isolated from a nipa-based bacterial display library. Curr Pharm Biotechnol. 2013; 14: 571-581. Ref.: https://goo.gl/eDJJTj
- Ferreira Junior A, Santiago FM, Silva MV, Ferreira FB, Macedo Junior AG, et al. Production, characterization and applications for toxoplasma gondii-specific polyclonal chicken egg yolk immunoglobulins. PloS One. 2012; 7. Ref.: https://goo.gl/OCvRCG
- Taghavian O, Spiegel H, Hauck R, Hafez HM, Fischer R, et al. Protective oral vaccination against infectious bursal disease virus using the major viral antigenic protein vp2 produced in pichia pastoris. PloS One. 2013; 8. Ref.: https://goo.gl/59SpWh
- Jakka P, Reddy YK, Kirubaharan JJ, Chandran ND. Evaluation of immune responses by live infectious bursal disease vaccines to avoid vaccination failures. Eur J Microbiol Immunol (Bp). 2014; 4: 123-127. Ref.: https://goo.gl/tkY50j
- Jain P, Singh R, Saxena VK, Singh KB, Ahmed KA, et al. In vitro rapid clearance of infectious bursal disease virus in peripheral blood mononuclear cells of chicken lines divergent for antibody response might be related to the enhanced expression of proinflammatory cytokines. Res Vet Sci. 2013; 95: 957-964. Ref.: https://goo.gl/3shwP7
- Dantas-Barbosa C, de Macedo Brigido M, Maranhao AQ. Antibody phage display libraries: Contributions to oncology. Int J Mol Sci. 2012; 13: 5420-5440. Ref.: https://goo.gl/RISxFI
- Tabll A, Abbas AT, El-Kafrawy S, Wahid A. Monoclonal antibodies: Principles and applications of immmunodiagnosis and immunotherapy for hepatitis c virus. World J Hepatol. 2015; 7: 2369-2383. Ref.: https://goo.gl/94E615
- Gonzalez D, Rodriguez JF, Abaitua F. Intracellular interference of infectious bursal disease virus. J Virol. 2005; 79: 14437-14441.Ref.: https://goo.gl/PMzQix
- Parker D, de Wit S, Houghton H, Prandini F. Assessment of impact of a novel infectious bursal disease (ibd) vaccination programme in breeders on ibd humoral antibody levels through the laying period. Vet Rec Open. 2014; 1. Ref.: https://goo.gl/BEB8bQ
- Thomson L, Tenopoulou M, Lightfoot R, Tsika E, Parastatidis I, et al. Immunoglobulins against tyrosine-nitrated epitopes in coronary artery disease. Circulation. 2012; 126: 2392-2401. Ref.: https://goo.gl/pJPE72
- Brady LJ. Antibody-mediated immunomodulation: A strategy to improve host responses against microbial antigens. Infect Immun. 2005; 73: 671-678. Ref.: https://goo.gl/idnj1K