Research Article
Photocatalytic Degradation of Microcystins-LR over Mesoporous graphitic Carbon Nitride (mpg-CN)

Laiyan Wu1, Jirong Lan1, Anping Yang3, Yanxi Zhao1, Songbo Wang1 and Junjiang Zhu1,2*
1Key Laboratory of Catalysis and Materials Science of the State Ethnic Affairs & Commission Ministry of Education, South-Central University for Nationalities, Wuhan 430074, China
2Institute of Catalysis for Energy and Environment, College of Chemistry and Chemical Engineering, Shenyang Normal University, Shenyang 110034, China
3Department of Testing and Analysis, Hubei Provincial Environmental Monitoring Center Station, Wuhan 430072, China
*Address for Correspondence: Dr. Junjiang Zhu, Key Laboratory of Catalysis and Materials Science of the State Ethnic Affairs & Commission Ministry of Education, South-Central University for Nationalities, Wuhan 430074, China, Email: [email protected]
Dates: Submitted: 14 April 2017; Approved: 22 May 2017; Published: 24 May 2017
How to cite this article: Wu L, Lan J, Yang A, Zhao Y, Wang S, et al. Photocatalytic Degradation of Microcystins-LR over Mesoporous graphitic Carbon Nitride (mpg-CN). Ann Adv Chem. 2017; 1: 012-22. DOI: 10.29328/journal.aac.1001002
Copyright License: © 2017 Wu L, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Mpg-CN; Microcystin-LR; Photo degradation; Reusability; Mechanism
ABSTRACT
Mesoporous graphitic carbon nitrides (mpg-CN) were synthesized by a templating method using Ludox (SiO2) as hard template and guanidine hydrochloride (GndCl) as precursor, and were used as metal-free photocatalysts for microcystin-LR (MC-LR) degradation in aqueous solution. By tuning the mass ratio of SiO2 to GndCl, mpg-CN with varied surface areas and condensation degrees were obtained. Catalytic results showed that sample prepared at mass ratio equals 0.4, i.e., mpg-CN(0.4), exhibits the best activity, with above 98% MC-LR conversion obtained at 120 min. Mechanism studies suggested that the reaction obeys the pseudo first-order equation and the produced superoxide anion radicals (•O2−) is the major reactive intermediates contributing to the reaction. Stability tests showed that no appreciable loss of activity is observed even the catalyst is recycled for five times, indicating that the material is stable in the reaction.
INTRODUCTION
Occurrence of cyanobacterial harmful algal blooms causes water deterioration, shortage as well as damage to the ecosystem [1], and is becoming a worldwide environmental problem. Microcystins (MCs) are monocyclic heptapetides produced by the freshwater cynobacteria, having adverse effects on aquatic animals and human beings via food chain [2]. Among the 90+ variants of the MCs, Microcystin-LR (MC-LR) is commonly detected and has the most toxic effect[1]. For this, the World Health Organization (WHO) has issued a guideline value of 1 μg L−1 MC-LR in drinking water and a tolerable daily intake (TDI) of 0.04 μg kg−1 body weight per day of MC-LR in aquatic product [3,4].
MCs are stable in natural water, and can last for months or even years at high temperatures (40°C) and extreme pH solution, in the presence of sunlight and/or enzymes [5,6], making them hard to be removed by the conventional physical and biological technologies. Advanced oxidation processes (AOPs) could be effective for their elimination in the presence of strong oxidants, such as chlorine, ozone, hydrogen peroxide [7-9], but the continuous input of expensive chemical reagents and recontaminations are prohibitive. Recently, the photocatalysis technology with UV/TiO2 system has been suggested to be a good strategy for the removal of organic pollutants in wastewaters. This system can exhibit fast, effective and environment-friendly properties in mineralizing the pollutants, by producing strongly oxidative electron/hole pairs, e.g.,h+,•O2− and •OH radicals [10-14]. However, TiO2-based photocatalysts require UV irradiation (λ<400nm) to activate the electrons, and less than 5% of the total solar energy could be utilized due to the large band gap (~3.2 eV) of TiO2, limiting its practical use. The use of other photocatalysts, instead of TiO2, with high utilization of solar energy is therefore needed.
Metal-free graphitic carbon nitride (g-CN) is a class of polymer material with semiconductor properties. The band gap of g-CN is 2.7 eV, smaller than that of TiO2, thus is more suitable to be used as photocatalyst with respect to increasing the utilization efficiency of solar energy. Indeed, this material has been found to be a promising catalyst in various catalytic applications including photocatalysis [15-17], due to its tailorable textural structure and rich surface chemistries. Also, the g-CN has been proved to be stable either in acidic, neutral or basic solution[18,19], thus could be an ideal catalyst for reactions conducted in aqueous solution under various environmental conditions, like that in wastewater. However, g-CN prepared by the polycondensation method normally exhibits low surface area (< 10 m2/g) because of its graphitic layered structure, limiting the catalytic performances. In order to improve the surface area and the catalytic performances of g-CN, the preparation of materials with porous structure is anticipated, which can be achieved using a templating method, with either hard or soft template [20,21]. Indeed, it is reported that mesoporous graphitic carbon nitride (mpg-CN) exhibits high surface area (∼200 m2/g) and shows improved photocatalytic performances in, for example, hydrogen production [22] and degradation of organic dyes [23].
The aim of this work is to study the photodegradation behaviors of MC-LR on mpg-CN and its reaction mechanism. There are lots of works discussing the catalytic performances of mpg-CN for photodegradation of organic in literature [24]. Relative to these reported organics, MC-LR is a biologics with bigger molecular weight, and is rather stable in water, and only few works on the application of mpg-CN for MC-LR photodegradation are reported in literature. Herein, we systematically investigated the reaction and its mechanism, to provide fundamental knowledge on the application of mpg-CN for photodegradation of biologic MC-LR in aqueous solution.
MATERIALS AND METHODS
Chemicals
MC-LR (>95% purity,Wako,Japan),methanol and acetonitrile with HPLC grade (TEDIA Company, Inc., Fairfield, OH, USA), formic acid (Merck,Germany), and ultra-pure water (Bedford, MA, USA) were purchased and used as received. Other reagents were purchased from sinopharm chemical reagent Co., Ltd. with analytical reagent grade.
Preparation of mpg-CN
4.0 g GndCl was added to 4 mL deionized water with stirring. After dissolution a certain amount of Ludox (namely, 5.7, 10.0 and 14.3g .The SiO2 dispersion is 28%) was added dropwise, corresponding to a mass ratio (r) of SiO2 to GndCl is 0.4, 0.7 and 1.0, respectively. The mixture was then heated at 50°C and after the water was evaporated, the resulting white solid was dried at 100°C overnight, heat-treated in N2 at 550°C for 3 h (ramping rate of 3°C min-1). The obtained samples were treated with 50 mL 4 M NH4HF2 for 48 h with drastic stirring to remove the silica template, and finally dried in an vacuum oven at 60°C for 2 h. Depending on the mass ratio of SiO2 to GndCl, the products were named as mpg-CN(r), where r=0.4, 0.7 and 1.0, respectively.
Characterization of catalysts
The samples were characterized by X-ray diffraction (XRD, Bruker D8 Advance X-ray diffractometer), Fourier transform infrared spectroscopy (FT-IR, Nicolet 470 FT-IR spectrometer), thermal gravimetric analysis (TGA, NETZSCH TG 209F3), transmission electron microscopy (TEM, Tecnai G2 20 S-Twin), X-ray photoelectron spectroscopy (XPS, VG Multi lab 2000), N2 physisorption (TriStar II 3020, Micromeritics, USA), temperature programmed desorption of CO2 (CO2-TPD, TP-5080 TPD/TPR apparatus, Tianjing Xianquan technology company) and diffuse reflectance spectrum (DRS) with UV-vis spectrophotometer (Lambda, Bio 35, PE Co., USA), which were described in detail in previous works [25,26].
Photocatalytic degradation of MC-LR
10 mL MC-LR suspensions and 5 mg mpg-CN were mixed and stirred at room temperature (which was controlled at 20°C by an air conditioner) for 60 min in the dark to achieve adsorption equilibrium. Thereafter a LED lamp (the wavelength centered mainly at 420±10 nm) was switched on to initiate the photocatalytic reaction, keeping stirring. During the reaction 0.5 mL of reaction mixture was withdrawn by a syringe at given intervals, which was centrifuged at 10000 rpm for 10 min and filtered with a 0.22 μm membrane filter before the HPLC analysis.
HPLC/MS analysis
A stock of MC-LR solution (10 mg L-1) was prepared using methanol as solvent. A standard calibration curve was made with MC-LR concentrations ranging from 5 to 1000 μg L-1. The determination of MC-LR was carried out on a HPLC-MS/MS system that consists of an Agilent 1290 HPLC and an Agilent 6460 mass spectrometer. The compositions of solution were analyzed with a Zorbax Eclipse plus C18 column (3.5µm, 3.0 x100mm) and the sample injection volume was 10 μL. The mobile phase consisted of 0.1% HCOOH aqueous solution (solvent A) and pure CH3OH (solvent B). A gradient elution was used, starting with water: methanol volume ratio of 80:20 from 0 to 5 min, and then ramping to 20:80 from 5.1 min, until returning to the original conditions to re-equilibrate the system. The gas (nitrogen) temperature and flow rate were set at 325°C and 6 L min-1. The sheath gas temperature and flow rate were set at 350°C and 11 L min-1. The instrument was operated in a positive ion mode. MC-LR were monitored by MRM mode, using 210 V fragmentor and 78 eV collision energy.
RESULTS AND DISCUSSION
Characterizations of the samples
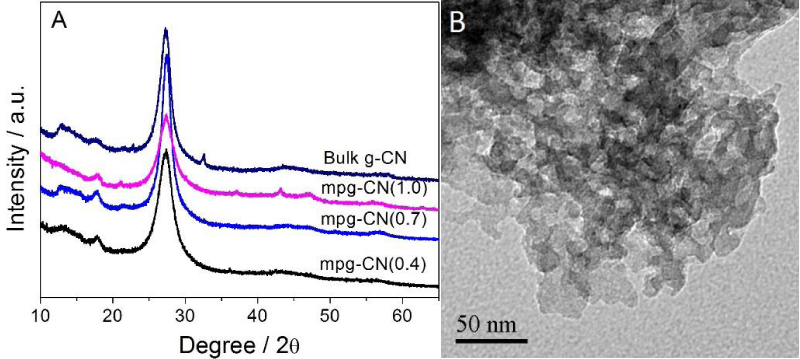
Figure 1A shows that a strong diffraction peak at 2θ=27.3°, attributed to the characteristic diffraction peak of g-CN, appears in the XRD patterns of the three mpg-CN(r) samples, indicating that they all have the graphitic g-CN layered structure. This is further confirmed by the FT-IR and XPS spectra, where the stretching and/or bending vibrations assignable to the C-N-C and N-H groups, and the specific binding energy assignable to the C-(N)3, N-C=N, C-N-C groups, are observed, respectively, see figure S1A-C.
Figure 1: (A) XRD patterns of the mpg-CN(r) and that of the bulk g-CN; (B) TEM image of mpg CN (0.4).
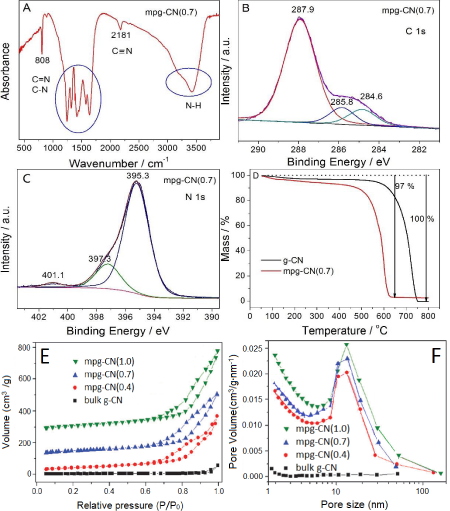
Figure S1: (A) FI-IR, (B) C 1s XPS spectrum, (C) N 1s XPS spectrum, (D) TGA curves of the specific mpg-CN(0.7) sample; (E) N2 physisorption isotherms and (F) pore size distributions of the bulk g-CN and mpg-CN(r) samples. Note: for simplicity we show here only the characterization result of sample at r=0.7 in the A-D picture.
With respect to the pore creation or the removal of silica template, TGA curves (figure S1D) show that less than 5% residues is left after the sample was heat-treated in air at 800°C, suggesting that the SiO2 template is mostly removed and pores are created. The creation of pores and the increase of surface area, from g-CN to mpg-CN(r), are supported by the N2 physisorption isotherms, which show that the surface area increase from 11 m2/g for g-CN to 144, 166 and 151 m2/g for r=0.4, 0.7 and 1.0, respectively, with average pore size of ca. 14 nm (figure S1E, 1F). It is noted that the surface area of the samples increase not linearly with the r increase, but reaches the maximum at r=0.7. This is explained by the thin wall of mpg-CN, thus it will be collapsed during the template removing process if higher r=10 is used, leading to decreased surface area. TEM image confirms further that pores with diameter of 16 nm are created in the samples, as seen in figure 1B. Detailed discussion on these characterizations can be found in a previous work [25] and are not going to describe here, to avoid repetition.
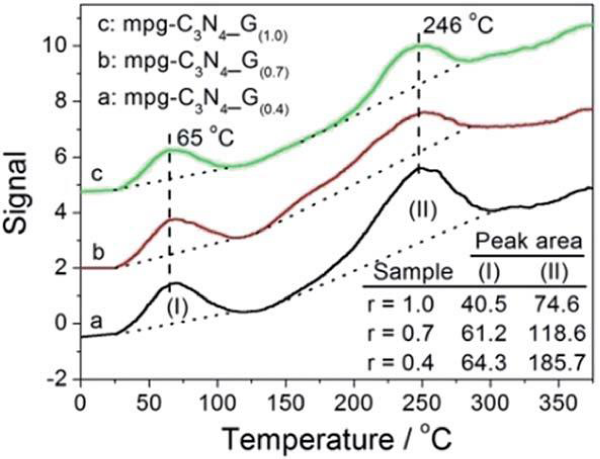
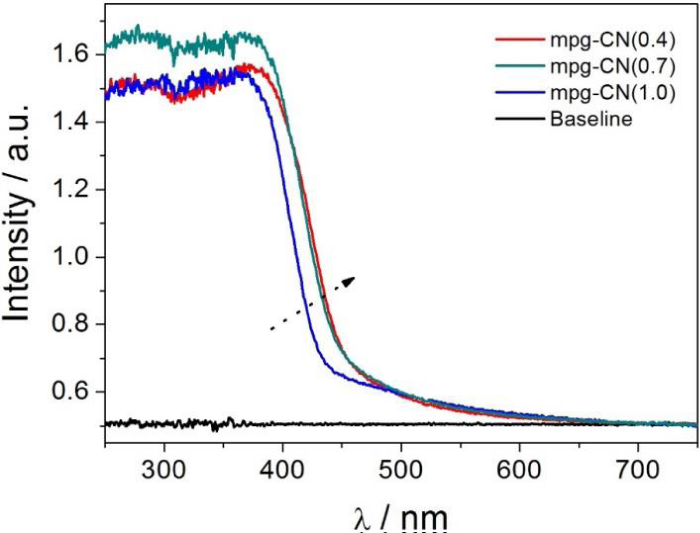
Besides the phase and textural structure, the surface chemistries of the samples were also evaluated, by a CO2-TPD measurement, and the results are presented in figure S2, which shows that mpg-CN (0.4) has the most, and mpg-CN (1.0) has the least amount of surface basic sites. It is known that g-CN is a base material, and the amount of surface basic sites depends intimately on the condensation degree of the sample. Sample with less condensation degree has more surface basic sites, and can absorb more CO2. Thus the large CO2 desorption peak of mpg-CN (0.4) suggests that it has a less condensed structure. On the other hand, it has been reported that the condensation degree of g-CN relates intimately to the energy of electron excitation, and sample with more condensed structure needs higher energy for excitation [24]. Thus, the absorption band edge in the UV-Vis spectra was red-shifted with the decrease of condensation degrees, from mpg-CN (1.0) to mpg-CN (0.7) and to mpg-CN (0.4), as depicted in figure 2.
Effect of SiO2 to GndCl mass ratio (r)
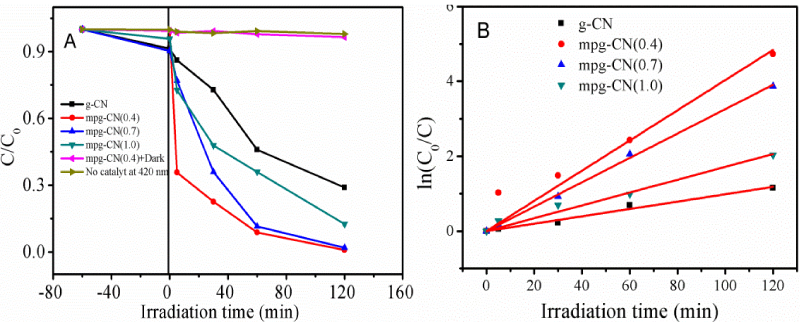
Figure 3A shows the photocatalytic activity of mpg-CN(r) for MC-LR degradation with 420 nm irradiation. For comparison, the photocatalytic activity of two blank experiments, without light irradiation or without catalyst, were also tested under identical experimental conditions. The blank tests show that MC-LR conversion is negligible in both cases, suggesting that the catalyst cannot catalyze the reaction without light irradiation and the reaction hardly occurs by direct photolysis. Therefore, the activity observed thereafter should be attributed to a photocatalysis process, which depends both on the catalyst and the light irradiation.
Figure 3: (A) Photodegradation activity of MC-LR over mpg-CN(r) and that obtained from blank experiments. (B) Pseudo-first-order kinetics of MC-LR degradation over the mpg-CN(r). Reaction conditions: 10 mL MC-LR (1.0 mg L-1), 5 mg catalysts, 420 nm irradiation.
The conversion increases abruptly when light irradiation and catalyst are simultaneously presented, in senquence of g-CN < mpg-CN (1.0) < mpg-CN (0.7) < mpg-CN (0.4). The increase of activity from g-CN to mpg-CN(r) can be attributed to the increased surface area and/or the creation of pores, which provide more surface to contact, and subsequently catalyze the degradation of substrate (MC-LR). For mpg-CN(r), the best activity is observed on mpg-CN (0.4). This can be attributed to its less condensed structure, enabling the electron excitation occurs at a relatively low light energy, as compared to that at r=0.7 and 1.0, see figure 2.
Kinetic calculation shows that the constant kapp increases abruptly from 0.98×10−2 min−1 for g-CN to 4.03×10−2 min−1 for mpg-CN(0.4), figure 3B, due to the improvement of surface area and the less condensed structure of mpg-CN(0.4). The well linear relationship between the ln(C0/C) and irradiation time (t) suggests that the reaction obeys the pseudo first-order equation. Because of the best activity, mpg-CN (0.4) is selected for the following experiments.
Effect of catalyst concentration
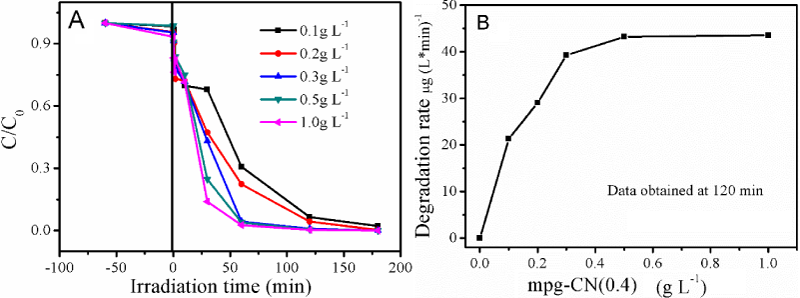
It was reported that photocatalysis is a catalyst mass dependent reaction [27]. Larger catalyst mass can produce more hydroxyl radicals and/or electrons by absorbing more photons, but excessive catalyst mass will reduce the opacity of suspension, blocking the energy from light radiation, thereby decreasing the degradation efficiency. To optimize the catalyst mass, we used five different catalyst concentrations (0.1, 0.2, 0.3, 0.5 and 1.0 g L-1) for investigation. Figure 4A shows the influence of catalyst concentration on the photocatalytic degradation activity of MC-LR. With the increase of catalyst concentration, from 0.1 to 0.5 g L-1, the degradation rate increases rapidly from 21.3 to 43.2 μg (L min)-1. But the further increase, from 0.5 to 1.0 g L-1, affects less on the degradation rate, which varied only from 43.2 to 43.5 μg (L min)-1, as shown in figure 4B, indicating that the optimum catalyst’s concentration of the present study is 0.5 g L-1.
Figure 4: ((A) Photocatalytic activity of mpg-CN (0.4) for MC-LR degradation as a function of irradiation time. (B) Effect of catalyst concentration on the degradation rate of MC-LR over mpg-CN (0.4). Reaction conditions: 10 mL 1.0 mg L-1 MC-LR, 420 nm irradiation.
Effect of initial MC-LR concentration
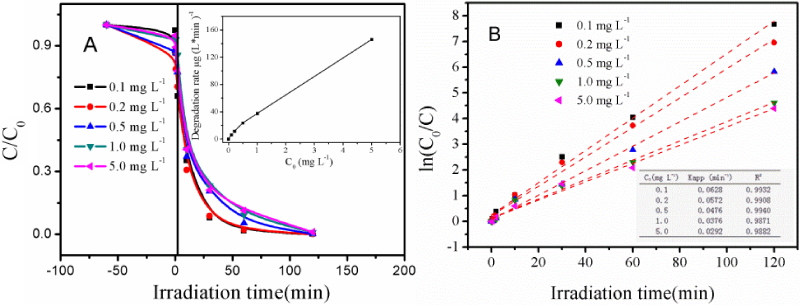
Figure 5A presents the photocatalytic activity of mpg-CN (0.4) for MC-LR degradation at different MC-LR initial concentrations. More than 75% MC-LR is degraded within 30 min and the reaction can be completed at 120 min, indicating that the catalyst is active and effective for MC-LR degradation within the investigated concentrations, from 0.1 to 5 mg L-1.
Figure 5: (A) Photocatalytic activity of mpg-CN (0.4) for MC-LR degradation as a function of irradiation time, obtained at different MC-LR initial concentrations. Insert: Variation of degradation rate versus initial concentrations. (B) Linear variation of ln(C0/C) versus irradiation time for MC-LR with different initial concentrations. Insert: Pseudo-first order apparent constant values obtained at different MC-LR initial concentrations.
The Langmuir-Hinshelwood (L-H) expression is a widely used method for the determination of relationship between the degradation rate and the substrate concentration in heterogeneous photocatalytic process, but it reflects only the “apparent” rate constant and reaction order [28,29]. To reveal the intrinsic relationship between them, pseudo-first order kinetics modified from the L-H expression is often used [30], as below.
Where kr is the reaction rate constant, Ks is the adsorption rate constant and kapp is the apparent constant, with the restriction of C=C0 at t=0, C0 being the initial concentration in the bulk solution after dark adsorption and t is the reaction time.
According to equation (2), the relationship between the degradation rate and the initial MC-LR concentration is plotted, as shown in the insert of figure 5A. The initial degradation rate increases from 6.28 to 145.95 μg (L min)-1 with increasing the MC-LR initial concentration from 0.1 to 5 mg L-1, and unlike the typical L-H model depicted [28,31], no platform appears in the profile, suggesting that the catalyst can be applied to higher MC-LR concentration. However, we did not try to study that further, as the MC-LR concentration in the real environment is far lower than the values studied herein [32,33].
Equation (2) can be further transformed into equation (3) after integration:
According to equation (3) and based on the data of figure 4A, the function between ln (C0/C) and irradiation time can be plotted, figure 5B, showing a well linear relationship between them, thus supports the assumption that the reaction obeys the pseudo first-order kinetics. The kapp, value, obtained from the regression analysis of the linear curve, decreases with increasing the MC-LR initial concentration. This can be explained by the limitation of active centers on the photocatalyst. Namely, with the addition of excessive MC-LR, the active center of the catalyst was mostly occupied (by the MC-LR and/or the produced intermediates), thereby reducing the solar light adsorption capability and leading to decreased reaction rate.
Reusability tests
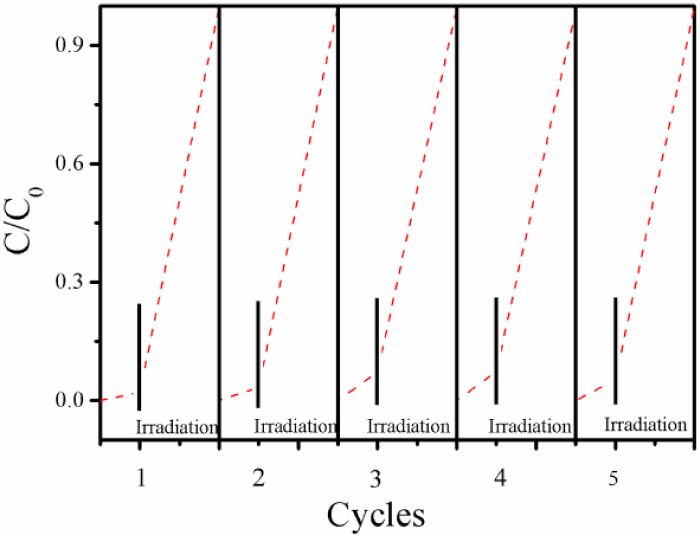
Figure 6 shows the reusability of mpg-CN (0.4) for photodegradation of MC-LR within five cycles. Before the light irradiation, the reaction mixture was stirred in dark for 60 min to ensure adsorption equilibrium. Less than 7% MC-LR was removed during this process, demonstrating that the activity observed thereafter is not due to the adsorption of MC-LR on the catalyst, but to the photocatalytic action. In the reusability tests, the catalyst was filtered and reused directly without any treatment. No appreciable decrease in the activity is observed and the activity profile is almost the same within the tests, indicating that the material is highly stable in the reaction and can be potential catalyst for MC-LR degradation in practical application.
Degradation mechanism
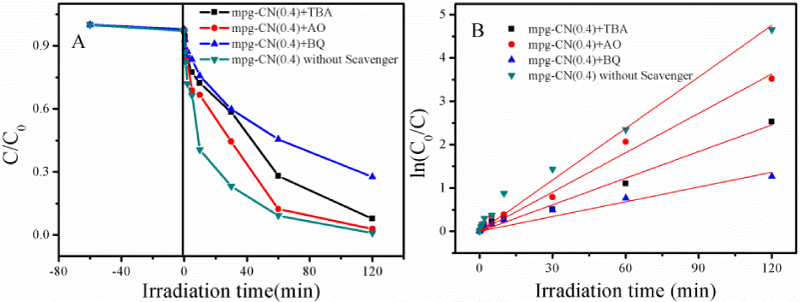
To study the reaction mechanism and inspect the reactive species influencing the reaction, quenching experiments were conducted. From literature we known that there are normally three reactive species responsible for the solar light induced photocatalytic reactions, namely, photogenerated holes (h+) [34,35], hydroxyl radicals (•OH) [36] and superoxide anion radicals (•O2−) [37]. To clarify their contributions to the reaction, three types of scavengers were added, including ammonium oxalate (AO, 1 mmol/L), tert-butanol (TBA,V (tBuOH:H2O)=1:20) and benzoquinone (BQ, 1 mmol/L), which are used to quench the h+, the •OH and the •O2− species, respectively.
Figure 7A shows that the addition of scavengers can influence the MC-LR photodegradation process, suggesting that the above three reactive intermediates contributes to the reaction. By comparison, it is found that the influence of the scavengers is in order of AO < TBA < BQ, suggesting that the •O2− species has the most, and the h+ species has the least contribution to the reaction, in sequence of •O2− > •OH > h+. That is, the •O2− species is the major factors accounting for the reaction. This is in accordance to previous results observed for the photodegradation of RhB and SMT conducted on g-CN [24,38]. Whereas, remember that the •OH radicals is a stronger oxidant than the •O2− species, the low contribution of •OH radicals must be that it is hard to produce or that the produced •OH radicals is quickly consumed before participating in the reaction. On the one hand, the direct generation of •OH radicals by hole oxidation (E0 (-OH/•OH)=2.4 V) is unfavorable due to the low valence band position of g-CN (1.4 V), and on the other hand, the produced •OH radicals will be consumed by the hydrogen of the surface basic -NH2 or =NH groups of g-CN [39,40]. This explains why the •OH species has less contribution to the reaction than that of the •O2− species.
Figure 7: Effect of scavengers on (A) Photodegradation activity of MC-LR over mpg-CN (0.4); (B) Pseudo-first-order kinetics of MC-LR degradation over mpg-CN (0.4).
The kinetics of MC-LR photodegradation on mpg-CN (0.4) in the absence and presence of scavengers are also calculated and shown in figure 7B. The kapp values obtained without the scavenger, and in the presence of TBA, AO and BQ are 0.03958, 0.02045, 0.03025, 0.01135 min-1, respectively. The well linear relationship between the ln(C0/C) and irradiation time supports that the reaction obeys the pseudo first order reaction, as discussed above.
CONCLUSIONS
Mesoporous graphitic carbon nitride (mpg-CN) was synthesized and applied as catalyst for photodegradation of MC-LR in aqueous solution. Results indicated that mpg-CN (0.4), synthesized at mass ratio of SiO2 to GndCl equals 0.4, shows the best photocatalytic activity for the reaction, with more than 75% MC-LR being degraded within 30 min and full degradation at 120 min, at reaction conditions of 0.5g L-1 mpg-CN (0.4) and 420 nm irradiation. In addition to the good photocatalytic activity, mpg-CN (0.4) is also highly stable for the reaction, thus no appreciable loss of activity was observed even after five runs. Mechanism studies suggested that reaction obeys the pseudo first-order equation, and the oxidation of MC-LR was conducted mainly via the superoxide anion radicals (•O2−). These results showed that mpg-CN(r) could have the potential application of treating MC-LR in water by photocatalysis.
ACKNOWLEDGMENT
Financial support from the National Science Foundation of China (21203254 and 21307164), the Natural Science Foundation of Hubei Province of China (2015CFA138), the environmental research program of Hubei Provincial Environmental Protection Bureau (2014HB08), “the Fundamental Research Funds for the Central Universities”, South-Central University for Nationalities (CZY17016)), the “Study Abroad for CSC Sponsored Chinese Citizen” and the “Academic Research Abroad from South-Central University for Nationalities Sponsored Outstanding Young Teacher”, is greatly appreciated.
REFERENCES
- Carmichael WW, Azevedo SM, An JS, Molica RJ, Jochimsen EM, et al. Human fatalities from cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ Health Persp. 2001; 109: 663-668. Ref.: https://goo.gl/STlMcB
- Chen J, Xie P, Li L, Xu J. First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage. Toxicol Sci. 2009; 108: 81-89. Ref.: https://goo.gl/mVlHbz
- Dietrich D, Hoeger S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol Appl Pharm. 2005; 203: 273-289. Ref.: https://goo.gl/Vp8cNV
- Falconer I, Bartram J, Chorus I, Kuiper-Goodman T, Utkilen H, et al. Safe levels and safe practices. In: Chorus i, bartram j (eds) toxic cyanobacteria in water-a guide to their public health consequences, monitoring and management. Published: E & FN Spon, 1999: 155-178.
- Tsuji K, Naito S, Kondo F, Ishikawa N, Watanabe MF, et al. Stability of microcystins from cyanobacteria: Effect of light on decomposition and isomerization. Environ Sci Technol. 1994; 28: 173-177. Ref.: https://goo.gl/MZX9im
- Harada K, Tsuji K, Watanabe MF, Kondo F. Stability of microcystins from cyanobacteria-iii. Effect of pH and temperature. Phycologia. 1996; 35: 83-88. Ref.: https://goo.gl/zwxaoN
- Miao H, Tao W. The mechanisms of ozonation on cyanobacteria and its toxins removal. Sep Purif Technol. 2009; 66: 187-193. Ref.: https://goo.gl/a6tsD4
- Pelaez M, Falaras P, Likodimos V, Kontos AG, de la Cruz KO AA, et al. Synthesis, structural characterization and evaluation of sol-gel-based NF-TiO2 films with visible light-photoactivation for the removal of microcystin-LR. Appl Catal B: Environ. 2010; 99: 378-387. Ref.: https://goo.gl/IfULFj
- Ho L, Onstad G, Von Guntern U, Rinck-Pfeiffer S, Craig K, et al. Differences in the chlorine reactivity of four microcystin analogues. Water Res. 2006; 40: 1200-1209. Ref.: https://goo.gl/ym7KE0
- Fotiou T, Triantis TM, Kaloudis T, Hiskia A. Evaluation of the photocatalytic activity of TiO2 based catalysts for the degradation and mineralization of cyanobacterial toxins and water off-odor compounds under UV-a, solar and visible light. Chem Eng J. 2015; 261: 17-26. Ref.: https://goo.gl/fiQaWU
- Andersen J, Han C, O’Shea K, Dionysiou DD. Revealing the degradation intermediates and pathways of visible light-induced NF-TiO2 photocatalysis of microcystin-LR. Appl Catal B: Environ. 2014; 154-155: 259-266. Ref.: https://goo.gl/S0riEM
- Vilela WFD, Minillo A, Rocha O, Vieira EM, Azevedo EB. Degradation of [D-Leu]-microcystin-LR by solar heterogeneous photocatalysis (TiO2). Sol Energy. 2012; 86: 2746-2752.
- Lawton LA, Robertson PKJ, Cornish BJPA, Jaspars M. Detoxification of microcystins (cyanobacterial hepatotoxins) using TiO2 photocatalytic oxidation. Environ Sci Technol. 1999; 33: 771-775. Ref.: https://goo.gl/kdc3YC
- Liu I, Lawton LA, Robertson PK. Mechanistic studies of the photocatalytic oxidation of microcystin-LR: An investigation of byproducts of the decomposition process. Environ Sci Technol. 2003; 37: 3214-3219. Ref.: https://goo.gl/RcQo96
- Wang XC, Maeda K, Thomas A, Takanabe K, Xin G, et al. Nat Mater, A metal-free polymeric photocatalyst for hydrogen production from water under visible light. 2009; 8: 76-80. Ref.: https://goo.gl/y7PFGV
- Wang XC, Blechert S, Antonietti M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012; 2: 1596-1606. Ref.: https://goo.gl/aF8l4X
- Xiang Q, Yu J, Jaroniec M. Preparation and enhanced visible-light photocatalytic H2-production activity of graphene/C3N4 composites. J Phys Chem C. 2011; 115: 7355-7363. Ref.: https://goo.gl/b1vZO7
- Wang Y, Wang X, Antonietti M. Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: From photochemistry to multipurpose catalysis to sustainable chemistry. Angew Chem Int Ed. 2012; 51: 68-89. Ref.: https://goo.gl/cfHKZS
- Su DS, Zhang J, Frank B, Thomas A, Wang XC, et al. Metal-free heterogeneous catalysis for sustainable chemistry. ChemSusChem. 2010; 3: 169-180. Ref.: https://goo.gl/cs3EJl
- Xu J, Wu H-T, Wang X, Xue B, Li Y-X, et al. A new and environmentally benign precursor for the synthesis of mesoporous g-C3N4 with tunable surface area. Phys Chem Chem Phys. 2013; 15: 4510-4517. Ref.: https://goo.gl/hp9Ns9
- Wang Y, Wang XC, Antonietti M, Zhang Y. Facile one-pot synthesis of nanoporous carbon nitride solids by using soft templates. Chemsuschem. 2010; 3: 435-439. Ref.: https://goo.gl/vR7PY4
- Wang XC, Maeda K, Chen X, Takanabe K, Domen K, et al. Polymer semiconductors for artificial photosynthesis: Hydrogen evolution by mesoporous graphitic carbon nitride with visible light. J Am Chem Soc. 2009; 131: 1680-1681. Ref.: https://goo.gl/cTDH1M
- Cui Y, Ding Z, Liu P, Antonietti M, Fu X, et al. Metal-free activation of H2O2 by g-C3N4 under visible light irradiation for the degradation of organic pollutants. Phys Chem Chem Phys. 2012; 14: 1455-1462. Ref.: https://goo.gl/47dvGf
- Xiao H, Zhu J, Thomas A. Graphitic carbon nitride for photocatalytic degradation of sulfamethazine in aqueous solution under simulated sunlight irradiation RSC Advances. 2015; 5: 105731-105734.
- Yang Q, Wang W, Zhao Y, Zhu J, Zhu Y, et al. Metal-free mesoporous carbon nitride catalyze the Friedel-Crafts reaction by activation of benzene. RSC Advances. 2015; 5: 54978-54984.
- Xiao H, Wang W, Liu G, Chen Z, Lv K, et al. Photocatalytic performances of g-C3N4 based catalysts for RhB degradation: Effect of preparation conditions. Appl Surf Sci. 2015; 358: 313-318. Ref.: https://goo.gl/A5097Z
- Parra S, Olivero J, Pulgarin C. Relationships between physicochemical properties and photoreactivity of four biorecalcitrant phenylurea herbicides in aqueous TiO2 suspension. Appl Catal B: Environ. 2002; 36: 75-85. Ref.: https://goo.gl/7fDSU0
- Malato S, Fernández-Ibáñez P, Maldonado MI, Blanco J, Gernjak W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal Today. 2009; 147: 1-59. Ref.: https://goo.gl/YlBIsM
- Bouzaida I, Ferronato C, Chovelon JM, Rammah ME, Herrmann JM. Heterogeneous photocatalytic degradation of the anthraquinonic dye, Acid Blue 25 (AB25): a kinetic approach. J Photochem Photobiol A: Chem. 2004; 168: 23-30. Ref.: https://goo.gl/523gPm
- Tennakone K, Tilakaratne CTK, Kottegoda IRM. Photomineralization of carbofuran by TiO2-supported catalyst. Water Res. 1997; 31: 1909-1912. Ref.: https://goo.gl/WVw6oz
- T Fotiou, TM Triantis, T Kaloudis, Hiskia A. Evaluation of the photocatalytic activity of TiO2 based catalysts for the degradation and mineralization of cyanobacterial toxins and water off-odor compounds under uv-a, solar and visible light. Chem Eng J. 2015; 261: 17-26. Ref.: https://goo.gl/N8NvCn
- Izaguirre G, Jungblut AD, Neilan BA. Benthic cyanobacteria (Oscillatoriaceae) that produce microcystin-LR, isolated from four reservoirs in southern california. Water Res. 2007; 41: 492-498. Ref.: https://goo.gl/JR6QTC
- Wang Q, Niu Y, Xie P, Chen J, Ma Z, et al. Factors affecting temporal and spatial variations of microcystins in gonghu bay of lake taihu, with potential risk of microcystin contamination to human health. Sci World J. 2010; 10: 1795-1809. Ref.: https://goo.gl/wm1ZrK
- Wang X, Utsumi M, Yang YN, Li DW, Zhao YX, et al. Degradation of microcystin-LR by highly efficient AgBr/Ag3PO4/TiO2 heterojunction photocatalyst under simulated solar light irradiation. Appl Surf Sci. 2015; 325: 1-12. Ref.: https://goo.gl/NdqB7n
- Chen SF, Liu W, Zhang HY, Yu XL. Photocatalytic decolorization of soluble dyes by a bis-ions coexistence system of NH4+ and NO3− with high photoreduction ability. J Hazard Mater. 2011; 186: 1687-1695. Ref.: https://goo.gl/lpPsq7
- Minero C, Mariella G, Maurino V, Vione D, Pelizzetti E. Photocatalytic transformation of organic compounds in the presence of inorganic ions. 2. Competitive reactions of phenol and alcohols on a titanium dioxide-fluoride system. Langmuir. 2000; 16: 8964-8972. Ref.: https://goo.gl/sYwRHC
- Yin MC, Li ZS, Kou J, Zou Z. Mechanism investigation of visible light-induced degradation in a heterogeneous TiO2/eosiny/rhodamine B system. Environ Sci Technol. 2009; 43: 8361-8366. Ref.: https://goo.gl/nBh8DT
- Fang S, Lv KL, Li Q, Ye HP, Du DY, et al. Effect of acid on the photocatalytic degradation of rhodamine B over g-C3N4. Appl Surf Sci. 2015; 358: 336-342. Ref.: https://goo.gl/w364ca
- Yan SC, Li ZS, Zou ZG. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir. 2009; 25: 10397-10401. Ref.: https://goo.gl/O1jVlX
- Cui YJ, Huang JH, Fu XZ, Wang XC. Metal-free photocatalytic degradation of 4-chlorophenol in water by mesoporous carbon nitride semiconductors. Catal Sci Technol. 2012; 2: 1396-1402. Ref.: https://goo.gl/zoHfiC