Research Article
The impact of geographical origin on specific properties of pine honey

Ioannis K Karabagias*, Christos Nikolaou and Ilias Gatzias
Laboratory of Food Chemistry, Department of Chemistry, University of Ioannina, Ioannina 45110, Greece
*Address for Correspondence: Dr. Ioannis K Karabagias, Department of Chemistry, Section of Industrial and Food Chemistry, Laboratory of Food Chemistry, University of Ioannina, Ioannina Campus, 45110, Greece, Email: [email protected]
Dates: Submitted: 02 June 2017; Approved: 28 June 2017; Published: 30 June 2017
How to cite this article: Karabagias IK, Nikolaou C, Gatzias I. The impact of geographical origin on specific properties of pine honey. Ann Adv Chem. 2017; 1: 023-031. DOI: 10.29328/journal.aac.1001003
Copyright License: © 2017 Karabagias IK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ABSTRACT
Pine honey represents the major type of honey produced in Greece. In that sense, the aim of the present study was to investigate if specific physicochemical and bioactive properties could serve as markers of its geographical origin. For this purpose, forty pine honey samples were collected during harvesting years 2011 and 2012 from Halkidiki and Thassos, the well-known pine honey producing areas in Greece. Physicochemical parameters taken into account, using conventional and literature cited methods, were: pH, CIE colour parameters L*,a*,b*, and browning index. Furthermore, colour intensity and the in vitro radical scavenging activity were estimated by the application of spectrometric assays. Results showed that, pine honeys exhibited statistically significant differences (p<0.05) in pH, colour intensity, and radical scavenging activity, depending on geographical origin. On the basis of radical scavenging activity results obtained, pine honeys proved to have a high in vitro antioxidant “character’’. Finally, perfect Pearson’s correlations (r=1) at the confidence level p<0.01 were obtained for the sets: pH-browning index, pH-radical scavenging activity, and browning index -radical scavenging activity, with respect to geographical origin.
INTRODUCTION
“Without honey this world would be less sweater’’, could be a few words to describe the importance of honey in the culture, food casualties, and history of many civilizations. It is produced from Apis mellifera honeybees either from nectar or honeydew secretions [1].
Pine honey (Pinus spp.) belongs to honeydew honeys and originates from honeydew secretions of the insect Marchalina hellenica [2]. It is the most common honey type in Greece, representing the 65% of the total annual production, which is estimated to be 13,000-15,000 tons [1]. Some typical producing regions in Greece include: Halkidiki, Thassos, Evia, Zakynthos, Skiathos, Rhodes, Skopelos and Crete.
Nowdays, there is great tendency at an international level, for products with unique chemical composition and characteristic properties. Such products hold a special position at the international market and gain, of course, a higher price. The scientific community has been focused on such a hot topic, involving several agricultural products, in terms of ‘’authentication’’ [3-5].
Due to its great variability in certain micronutrients such as minerals, vitamins, organic acids, flavonoids, phenolic acids, enzymes, and other phytochemicals, honey possesses a considerable antioxidant activity [6]. This property, at routine control, is usually measured in vitro, by reproducible spectrometric assays [7,8].
However, it should be stressed that, the quantity of these antioxidant components along with specific physicochemical parameters (i.e. pH, colour, acidity, etc.), varies widely according to botanical and geographical origin of honey [4,8-11]. In addition, processing, handling and storage of honey may influence its composition, and thus, its properties [9,12-14].
Based on the above, the aim of the present study was to investigate whether geographical origin could affect pH, colour parameters (L*, a*, b*), browning index, colour intensity, and in vitro radical scavenging activity. To the best of our knowledge, data on these specific parameters involving Halkidiki and Thassos regions has not been previously reported. To avoid misleading, it should also be stressed that, experimental data may be generalized at a global research level, and do not involve only the domestic scientific community [4].
MATERIALS AND METHODS
Materials
Honey samples: Forty (N=40) unifloral pine honey samples (Pinus spp.) were donated from Attiki Bee Culturing Co. Alex. Pittas S.A, during the harvesting years 2011 and 2012. Honey samples were collected from the major pine production areas in Greece, namely Halkidiki (N=20) (coordinates: 40°20′N 23°30′E) and Thassos (N=20) (coordinates: 40°41′N 24°39′E). In order to ensure the botanical origin of pine honey samples, the melissopalynological analysis was applied according to a previous work [1]. All honey samples were stored in glass containers, shipped to the laboratory, and maintained at 4±1 °C until analyses.
Reagents and solutions: 2,2-Diphenyl-1-picrylhydrazyl (DPPH), was purchased from Sigma-Aldrich (Germany). Methanol and acetate buffer (CH3COONa*3H2O) were purchased from Merck (KGaA, 64271, Darmstadt, Germany).
Methods
pH measurements: The pH was measured using a Delta OHM, model HD 3456.2, pH-meter (Padova, Italy) with a precision of ± 0.002 pH units in a solution of 10 g honey in 75 mL of CO2 free distilled water [15]. All measurements were performed in duplicate (n=2).
Determination of CIE colour parameters
CIE colour parameter values L*, a*, and b*, were measured in an aqueous honey solution [1]. In the CIE L*a*b* uniform colour space, the colour coordinates are: L* (lightness), a*(red/green coordinate), with +a* indicating red, and -a* indicating green; and b* (yellow/blue coordinate), with +b* indicating yellow, and -b* indicating blue. The L*, a*, and b* coordinate axis defines the three dimensional CIE colour space. Thus, if the L*, a*, and b* coordinates are known, colour is located in a quadrant [16]. Every value is the average of six determinations (n=6).
Determination of browning index values
The browning index (BI) was calculated using L*, a*, b* values according to Kortei et al. [16]: BI = [100 (X- 0.31)]/0.17 (Eq. 1),
Where: X = (a* + 1.75 L*) / (5.645 L* + a* - 3.012 b*).
BI represents the purity of brown colour and is considered as an important parameter associated with browning effects. For example, thermal treatment causes browning development in honey. Every value is the average of six determinations (n=6).
Determination of colour intensity: ABS450
Honey was diluted to a 50% (w/v) with warm water (fixed temperature 45°C) (AREX Heating Magnetic Stirrer, VTF Digital Thermoregulator, VELP Scientifica, Via Stazione, 16, 20865 Usmate Velate Monza e Brianza, Italy) sonicated (Elma, Elmasonic model S 10H, Germany) for 5 min and filtered using Whatman filters (CAT. No. 6780-2504, UK) with a pore size of 0.45 μm, to remove any solid particles. The net absorbance was defined as the difference between spectrometric absorbance at 450 and 720 nm. The colour intensity (ABS450) of honey, evaluates the contribution of coloured phytochemicals (carotenoids, flavonoids) to the development of a distinguishable colour. Results were expressed as mAU [8]. Every value is the average of three determinations (n=3).
In vitro estimation of radical scavenging activity (% RSA)
a) Preparation of [DPPH·] free radical standard solution
A standard solution of [DPPH·] 1.12x10−4 mol/L (M) was prepared by dissolving 0.0044 g of the free radical [DPPH·] in 100 mL methanol according to Karabagias et al. [8].
b) Preparation of [DPPH·] free radical calibration curve
A calibration curve of concentration versus absorbance of DPPH was prepared using methanol, according to Karabagias et al. [8]. Parameters such as the % decrease in [DPPH·] free radical absorbance (% RSA), % decrease in [DPPH·] free radical concentration, % [DPPH·] remaining of the mixture obtained by the addition of honey solution when the reaction reached plateau (4h), were estimated by the above calibration curve [8].
c) Determination of radical scavenging activity (% RSA)
The radical scavenging activity of aqueous pine honey solutions was calculated in vitro using the DPPH assay according to the method of Karabagias et al. [8]. The absorbance of the reaction mixture was measured at 517 nm after 4h, in a UV/VIS Spectrometer (PerkinElmer, Lambda 25, USA).
[DPPH•] acts as a monitor/screener of chemical reactions involving radicals, while it serves as a common antioxidant assay [7,8]. Therefore, the reduction rate of a chemical reaction upon addition of [DPPH•], is used as an indicator of the radical nature of that reaction. Because of a strong absorption band centered at 517 nm, the [DPPH•] radical has a deep violet color in solution and it becomes colorless or pale yellow, when neutralized, by the action of i.e. antioxidant components (Figure 1).
The [DPPH·] radical scavenging activity was calculated using the following equation: %RSA= A0-At/A0 x 100 (Eq.2), where A0 is the initial absorbance of the [DPPH·] free radical standard solution and at is the absorbance of remaining [DPPH·] free radical after reaction with honey water soluble antioxidants, at steady state (t, plateau=4h). For this antioxidant test, methanol and acetate buffer (2:1, v/v) were used as the blank. Each analysis was run in triplicate (n=3).
STATISTICAL ANALYSIS
One sample t-test was applied to the average values of the investigated parameters, in order to test the impact of geographical origin, at the confidence level p<0.05. Correlations were obtained by Pearson’s correlation coefficient (r), at the confidence level p<0.05. Statistical treatment of data was accomplished using the SPSS v.22.0 statistics software.
RESULTS
Melissopalynological analysis
Fungal spores from Coleosporium were found in all pine honey samples from Halkidiki and Thassos. Greek pine honeys had a relatively few honeydew elements and large numbers of pollen grains, which resulted in a honeydew element per pollen grain ratio (HDE/P) <3. Present results are typical of Greek pine honeys based on previously reported studies [17].
Physicochemical parameter analysis
In tables 1 and 2, are shown full data regarding the parameters determined in pine honeys, according to geographical origin.
| Table 1: Physicochemical properties of pine honeys from Thassos. | ||||||||
| Geographical origin | Harvesting year | pH | L* | a* | b* | BI | Colour Intensity | % RSA |
| Thassos | 2011 | 4.81 | 71.33 | -3.04 | 13.35 | 16.98 | 399 | 39.14 |
| Thassos | 2011 | 5.10 | 71.76 | -4.33 | 19.02 | 15.47 | 302 | 29.92 |
| Thassos | 2011 | 4.49 | 69.46 | -3.65 | 22.52 | 16.81 | 564 | 35.66 |
| Thassos | 2011 | 4.48 | 69.19 | -3.69 | 22.88 | 16.84 | 569 | 38.73 |
| Thassos | 2011 | 4.46 | 67.89 | -3.40 | 23.00 | 17.53 | 545 | 50.41 |
| Thassos | 2011 | 4.46 | 69.92 | -3.44 | 20.22 | 16.92 | 526 | 50.82 |
| Thassos | 2011 | 4.46 | 69.08 | -3.82 | 24.51 | 16.72 | 528 | 48.98 |
| Thassos | 2011 | 4.46 | 69.07 | -3.44 | 23.53 | 17.16 | 575 | 36.88 |
| Thassos | 2011 | 4.46 | 70.61 | -4.06 | 19.99 | 16.06 | 588 | 45.91 |
| Thassos | 2011 | 4.43 | 68.74 | -3.36 | 23.60 | 17.34 | 500 | 37.50 |
| Thassos | 2012 | 4.96 | 60.94 | -1.23 | 40.08 | 22.59 | 690 | 48.77 |
| Thassos | 2012 | 5.09 | 62.67 | -2.69 | 36.56 | 20.08 | 647 | 53.48 |
| Thassos | 2012 | 4.67 | 62.13 | -2.03 | 36.17 | 21.10 | 572 | 60.46 |
| Thassos | 2012 | 5.58 | 69.99 | -4.83 | 29.16 | 15.35 | 493 | 44.26 |
| Thassos | 2012 | 5.03 | 63.90 | -3.25 | 35.66 | 18.96 | 329 | 60.25 |
| Thassos | 2012 | 4.82 | 67.50 | -3.74 | 21.28 | 17.25 | 668 | 36.88 |
| Thassos | 2012 | 4.80 | 67.73 | -3.78 | 23.28 | 17.14 | 360 | 40.37 |
| Thassos | 2012 | 4.78 | 69.29 | -4.94 | 24.16 | 15.40 | 389 | 47.75 |
| Thassos | 2012 | 4.81 | 63.04 | -2.45 | 35.03 | 20.24 | 492 | 51.84 |
| Thassos | 2012 | 4.82 | 62.21 | -1.75 | 36.65 | 21.42 | 595 | 71.72 |
| Average | 4.75 | 67.32 | -3.35 | 26.53 | 17.87 | 516.55 | 46.49 | |
| ±SD | 0.30 | 3.45 | 0.95 | 7.48 | 2.12 | 110.28 | 10.16 | |
| Comparison of average values by one sample t-test (p<0.05). SD: standard deviation. | ||||||||
| Table 2: Physicochemical properties of pine honeys from Halkidiki Comparison of average values by one samplet-test (p<0.05). SD: standard deviation. | ||||||||
| Geographical origin | Harvesting year | pH | L* | a* | b* | BI | Colour Intensity | % RSA |
| Halkidiki | 2011 | 5.08 | 71.30 | -3.58 | 14.28 | 16.40 | 281 | 60.71 |
| Halkidiki | 2011 | 5.27 | 68.74 | -3.54 | 16.87 | 17.14 | 328 | 71.69 |
| Halkidiki | 2011 | 4.94 | 65.90 | -3.46 | 22.38 | 18.06 | 196 | 69.83 |
| Halkidiki | 2011 | 4.42 | 72.20 | -5.06 | 19.36 | 14.58 | 289 | 65.16 |
| Halkidiki | 2011 | 4.60 | 60.79 | -1.95 | 30.03 | 21.74 | 252 | 55.19 |
| Halkidiki | 2011 | 4.97 | 69.80 | -4.32 | 21.42 | 15.98 | 488 | 58.01 |
| Halkidiki | 2011 | 4.85 | 68.91 | -3.70 | 18.79 | 16.91 | 432 | 51.56 |
| Halkidiki | 2011 | 4.93 | 70.61 | -3.85 | 16.52 | 16.29 | 273 | 60.12 |
| Halkidiki | 2011 | 4.42 | 72.75 | -4.95 | 14.89 | 14.58 | 317 | 79.59 |
| Halkidiki | 2011 | 4.92 | 70.60 | -3.91 | 15.97 | 16.22 | 365 | 34.54 |
| Halkidiki | 2012 | 4.73 | 59.21 | -0.36 | 40.64 | 24.48 | 460 | 62.84 |
| Halkidiki | 2012 | 4.69 | 64.57 | -2.95 | 32.35 | 19.10 | 281 | 59.34 |
| Halkidiki | 2012 | 4.85 | 62.70 | -2.07 | 37.35 | 20.84 | 328 | 67.47 |
| Halkidiki | 2012 | 5.78 | 69.26 | -6.12 | 33.37 | 14.07 | 196 | 68.96 |
| Halkidiki | 2012 | 5.04 | 65.15 | -2.41 | 32.04 | 19.55 | 289 | 69.27 |
| Halkidiki | 2012 | 4.71 | 64.74 | -3.45 | 31.91 | 18.44 | 252 | 75.06 |
| Halkidiki | 2012 | 4.94 | 58.25 | 0.95 | 44.55 | 26.66 | 488 | 73.03 |
| Halkidiki | 2012 | 4.80 | 60.42 | -0.36 | 39.07 | 23.92 | 432 | 76.86 |
| Halkidiki | 2012 | 4.81 | 64.47 | -3.24 | 33.58 | 18.78 | 273 | 79.59 |
| Halkidiki | 2012 | 4.75 | 63.87 | -0.75 | 36.07 | 22.00 | 365 | 77.01 |
| Average | 4.88 | 66.21 | -2.95 | 27.57 | 18.79 | 329.25 | 65.79 | |
| ±SD | 0.30 | 4.48 | 1.77 | 9.77 | 3.52 | 89.73 | 11.01 | |
| The impact of geographical origin on pH values of pine honeys | ||||||||
The impact of geographical origin on pH values of pine honeys
pH recorded variations among samples of the same and different geographical origin. In general, pine honeys from Thassos region recorded lower pH values than those from Halkidiki region. Application of statistical analysis showed that pH varied significantly according to geographical origin (p<0.05).
The impact of geographical origin on CIE L*, a*, and b* colour values of pine honeys
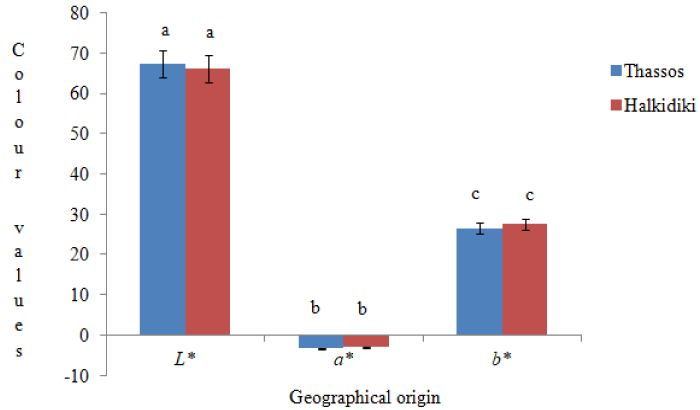
Results showed that pine honeys from Thassos and Halkidiki regions had green and yellow components (pigments). Furthermore, there were recorded variations in L*, a*, and b* values among samples of the same and different geographical origin. Application of statistical analysis showed that L*, a*, and b* colour parameters did not vary significantly according to geographical origin (p>0.05) (Figure 2).
Figure 2: Variations in L*, a*, and b* colour parameters according to geographical origin. Same lower case letters indicate no statistically significant differences (p>0.05).
The impact of geographical origin on browning index values of pine honeys
Results showed that pine honeys from Thassos region had lower browning index values than those from Halkidiki region. However, the observed differences were not statistically significant (p>0.05).
The impact of geographical origin on colour intensity values of pine honeys
Results showed that, there were recorded variations in colour intensity among samples of the same and different geographical origin. In particular, pine honey samples from Thassos region had higher colour intensity values than those from Halkidiki region. It should be stressed that, colour intensity reflects the content of pigments, the most important of which are polyphenols, carotenoids, xanthophylls and anthocyanins, that are grouped in water soluble and lipid soluble pigments. These components have been related with considerable antioxidant properties and are characteristic of honey botanical origin [7-9]. Statistical analysis showed that these differences were significant (p<0.05).
The impact of geographical origin on radical scavenging activity values (%RSA) of pine honeys
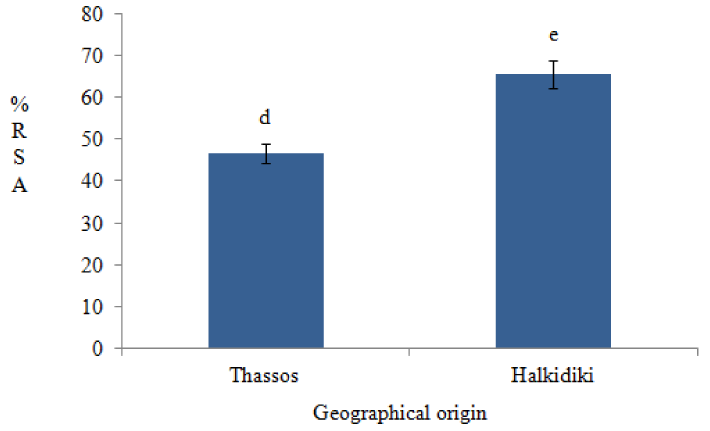
Results showed that, there were recorded variations in %RSA values among samples of the same and different geographical origin. More specifically, pine honey samples from Thassos region had lower %RSA values than those from Halkidiki region. Statistical analysis showed that these differences were statistically significant (p<0.001) (Figure 3).
Figure 3: Variations in radical scavenging activity (%RSA) of pine honeys according to geographical origin. Different lower case letters indicate statistically significant differences (p<0.001).
DISCUSSION
pH of honey ranges between 3.40 and 6.40. Such values are low enough to inhibit microorganisms’ development [18]. In general, light blossom or nectar honeys have lower pH values than honeydew honeys, due to their lower mineral content [19], Furthermore, it should be stressed that pH values were in agreement with the results reported by Terrab et al. [10] and Eleazu et al. [19], involving Moroccan honeydew and dark coloured Nigerian honeys, respectively. Finally, pH values have been previously reported to be an effective tool for geographical and botanical differentiation of honeydew honeys [1,10].
Colour is a physical property that is immediately apprehended by consumers. In different types of honey, varies from colourless and light yellow, to dark amber or nearly black, sometimes with green or reddish notes [20]. It is closely related to botanical origin, climate and soil conditions. Some authors have reported that pollen, sugars, carotenoids, xanthophylls, anthocyanins, minerals, amino acids, flavonoids, may all influence honey colour [21]. Commission Internationale de l’ Eclairage (CIE) system uses parameters L*, a*, and b*, to evaluate colour in numerous foodstuffs. The main advantage of this application is that stimulates the human eye observation when irritated with a specific colour.
In some countries, honey colour influences its price, according to consumers’ preferences and support. Generally, light honeys tend to have higher prices in the market, but there are some countries such as Germany, Switzerland, Greece and Turkey where dark honeydew honeys are preferably chosen [22].
L* values were much higher as compared to the work of Escriche et al. [14], involving honeydew honeys from Spain. In addition, present L* values are higher as compared to those obtained from European honeydew honeys [22]. However, a* and b* colour parameters of Greek pine honeys were much lower as compared to the aforementioned honeydew honeys [14,22]. In addition, colour parameters have been widely used for the geographical differentiation of honeydew honeys [1,22].
Components that could affect darkening are sugars, nitrogen content, free amino acids and moisture [23]. Browning index, serves as a qualitative criterion that indicates the existence or not of honey thermal processing. If honey is subjected to thermal treatment, the browning index increases with a high rate, due to the formation of non-enzymatic browning during the Maillard reaction [13]. On the other hand, the Maillard reaction depends on a great extent, on sugar and amino acids content, under thermal conditions. Besides, caramelization of sugars or presence of heat sensible compounds during thermal processing may increase BI or develop a dark-brown colour in honey [24]. Present results showed that pine honeys were not thermally treated, since browning index values were much lower as compared to thermal or ultrasound treated commercial honeys [25]. This comprises an additionally quality criterion for Greek pine honeys.
The high colour intensity values obtained for pine honeys are in agreement with those reported for Italian and Slovenian honeydew honeys, respectively [7,11].
The great variability on the content of certain honey micronutrients such as phenolic acids, flavonoids, carotenoids, organic acids, free amino acids, etc., which are documented to have antioxidant activity, results in a considerable variation of radical scavenging activity values, according to geographical and botanical origin. It should also be stressed that, dark coloured honeys possess higher antioxidant activity [7,8,14,26,27].
Pine honeys from Halkidiki recorded radical scavenging activity values in the same range with honeydew honey from Spain [26]. Escriche et al. [14], reported higher radical scavenging activity values for Spanish honeydew honeys, than those of the present study. More recently Sousa et al. [27], documented that Melliponi subnitida Ducke honeys from Brazil, possessed considerable radical scavenging activity. The reported values are in excellent agreement with those of the present study, involving pine honeys from Thassos region.
At this point, it should be very important to discuss the perfect Pearson’s correlations (r=1) (p<0.01) obtained for pH vs. BI, pH vs. %RSA, and BI vs. %RSA, with respect to geographical origin of pine honey. Results showed that, by increasing pH, browning index and radical scavenging activity values were increased. The same holds, for browning index values vs. radical scavenging activity values. This is in excellent agreement with the results reported by Turkmen et al. [13] and Escriche et al. [14], eventhough pine honey samples were not subjected to thermal treatment.
Regarding the effectiveness of higher pH values on the increasing trend of %RSA values, it should be proposed that a pH increase in the alkaline region, may favorably act as a medium for the isolation, dimerization, autoxidation, and structure transformation of flavonoids and related compounds that possess antioxidant activity [6].
As an executive summary, results of the present study clearly showed that production area of pine honey has a strong impact on specific physicochemical (pH, colour intensity) and bioactive properties (radical scavenging activity) (p<0.05). These differences may be of great interest to both: honey researchers and food industry (producers, exporters, etc.) at domestic and international levels, in terms of authentication and beneficial honey processing practices.
ACKNOWLEDGMENT
The authors are grateful to Attiki Bee Culturing Co., Alex. Pittas S.A, Protomagias 9, Kryoneri 14568, Athens, Greece, for the donation of pine honey samples and to Dr. Sofia Karabournioti for her assistance in the melissopalynological analysis. We also thank Professor Michael G. Kontominas, who provided access to the Laboratory of Food Chemistry, Department of Chemistry, University of Ioannina.
REFERENCES
- Karabagias IK, Badeka A, Kontakos S, Karabournioti S, Kontominas MG. Characterisation and classification of Greek pine honeys according to their geographical origin based on volatiles, physicochemical parameters and chemometrics. Food Chem. 2014; 146: 548-557. Ref.: https://goo.gl/x4S3BR
- Santas L. Insects producing honeydew exploited by bees in Greece. Apidologie. 1983; 14: 93-103. Ref.: https://goo.gl/Gcc8HY
- Granato D, de Oliveira CC, Fernandes-Caruso MS, Farah-Nagato LA, Alaburda J. Feasibility of different chemometric techniques to differentiate commercial Brazilian sugarcane spirits based on chemical markers. Food Res Int. 2014; 60: 212-217. Ref.: https://goo.gl/YSfyR3
- Karabagias IK, Louppis PA, Karabournioti S, Kontakos S, Papastephanou C, et al. Characterization and geographical discrimination ofcommercialCitrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017; 217: 445-455. Ref.: https://goo.gl/mfbn2z
- Locher C, Neumann J, Sostaric T. Authentication of honeys of different floral origins via high-performance thin-layer chromatographic fingerprinting. J Planar Chrom. 2017; 30: 57-62. Ref.: https://goo.gl/4wbUaJ
- Escuredo O, Míguez M, Fernández-González M, Seijo MC. Nutritional value and antioxidant activity of honeys produced in a European Atlantic area. Food Chem. 2013; 138: 851-856. Ref.: https://goo.gl/UWR1Cm
- Beretta G, Granata P, Ferrero M, Orioli M, Facino RM. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal Chim Acta. 2005; 533: 185-191. Ref.: https://goo.gl/7XaY68
- Karabagias IK, Dimitriou E, Kontakos S, Kontominas MG. Phenolic profile, colour intensity, and radical scavenging activity of Greek unifloral honeys. Eur Food Res Technol. 2016; 242: 1201-1210. Ref.: https://goo.gl/kzPS3L
- Gheldof N, Wang XH, Engeseth NJ. Identification and quantification of antioxidant components of honeys from various floral sources. J Agric Food Chem. 2002; 50: 5870-5877. Ref.: https://goo.gl/oDpgAM
- Terrab A, Díez MJ, Heredia FJ. Characterisation of Moroccan unifloral honeys by their physicochemical characteristics. Food Chem. 2002; 79: 373-379. Ref.: https://goo.gl/6C9QJM
- Bertoncelj J, Dobersek U, Jamnik M, Golob T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007; 105: 822-828. Ref.: https://goo.gl/9vujHe
- Wang XH, Gheldof N, Engeseth NJ. Effect of processing and storage on antioxidant capacity of honey. J Food Sci. 2004; 69: 96-101. Ref.: https://goo.gl/H99fGt
- Turkmen N, Sari F, Poyrazoglu ES, Velioglu YS. Effects of prolonged heating on antioxidant activity and colour of honey. Food Chem. 2006; 95: 653-657. Ref.: https://goo.gl/QtoHtm
- Escriche I, Kadar M, Juan-Borrás M, Domenech E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014; 142: 135-143. Ref.: https://goo.gl/NqFRwn
- IHC (Harmonized methods of the International Honey Commission). IHC Responsible for the methods: Stefan Bogdanov. Liebefeld, CH-3003 Bern, Switzerland: Swiss Bee Research Centre FAM. 1997.
- Kortei NK, Odamtten GT, Obodai M, Appiah V, Akonor PT. Determination of color parameters of gamma irradiated fresh and dried mushrooms during storage. Croat J Food Technol, Biotechnol Nutr. 2015; 10: 66-71.
- Dimou M, Katsaros J, Tzavella-Klonari K, Thrasyvoulou A. Discriminating pine and fir honeydew honeys by microscopic characteristics. J Apic Res Bee World. 2006; 45: 16-21. Ref.: https://goo.gl/tpRMLT
- Gomes S, Dias LG, Moreira LL, Rodrigues P, Estevinho L. Physicochemical, microbiological and antimicrobial properties of commercial honeys from Portugal. Food Chem Toxicol. 2010; 48: 544-548. Ref.: https://goo.gl/R5UjnW
- Eleazu CO, Iroaganachi MA, Okoronkwo JO. Determination of the Physico-Chemical Composition, Microbial Quality and Free Radical Scavenging Activities of Some Commercially Sold Honey Samples in Aba, Nigeria: ‘The Effect of Varying Colors’. Iran J Basic Med Sci. 2013; 16: 731-742. Ref.: https://goo.gl/oApSEP
- Eteraf-Oskouei T, Najafi M. Traditional and modern uses of natural honey in human diseases: a review. Iran J Basic Med Sci. 2013; 16(6): 731-742. Ref.: https://goo.gl/izkcmp
- Almeida-Muradian LB, Stramm KM, Estevinho LM. Efficiency of the FTIR ATR spectrometry for the prediction of the physicochemical characteristics of Melipona subnitida honey and study of the temperature’s effect on those properties. Int J Food Sci Technol. 2014;49: 188-195. Ref.: https://goo.gl/VX4YAd
- Tuberoso CIG, Jerković I, Sarais G, Congiu F, Marijanović Z, et al. Color evaluation of seventeen European unifloral honey types by means of spectrophotometrically determined CIE L* Cab* hab° chromaticity coordinates. Food Chem. 2014; 145: 284-291. Ref.: https://goo.gl/myJg1m
- Gonzales AP, Burin L, Buera MP. Color changes during storage of honeys in relation to their composition and initial color. Food Res Int. 1999; 32: 185-191. Ref.: https://goo.gl/Ye7Dje
- Can Z, Yildiz O, Sahin H, Turumtay EA, Silici S, et al. An investigation of Turkish honeys: their physico-chemical properties, antioxidant capacities, and phenolic profiles. Food Chem. 2015; 180: 133-141. Ref.: https://goo.gl/hURgAZ
- Chaikham P, Kemsawasd V, Apichartsrangkoon A. Effects of conventional and ultrasound treatments on physicochemical properties and antioxidant capacity of floral honeys from Northern Thailand. Food Biosci. 2016; 15: 19-26. Ref.: https://goo.gl/gBzeBq
- Vela L, de Lorenzo C, Pérez RA. Antioxidant capacity of Spanish honeys and its correlation with polyphenol content and other physicochemical properties. J Sci Food Agric. 2007; 87: 1069-1075. Ref.: https://goo.gl/Kzr4mY
- Sousa JM, de Souza EL, Marques G, Meireles B, de Magalhães AT, et al. Polyphenolic profile and antioxidant and antibacterial activities of monofloral honeys produced by Meliponini in the Brazilian semiarid region. Food Res Int. 2016; 84: 61-68. Ref.: https://goo.gl/BKFpEX