More Information
Submitted: June 11, 2022 | Approved: June 17, 2022 | Published: June 20, 2022
How to cite this article: Ali AH. High-Performance Liquid Chromatography (HPLC): A review. Ann Adv Chem. 2022; 6: 010-020.
DOI: 10.29328/journal.aac.1001026
Copyright License: © 2022 Ali AH. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Column; Mobile phase; Separation; Stationary phase
High-Performance Liquid Chromatography (HPLC): A review
Abdu Hussen Ali*
Chemistry Department, College of Natural and Computational Sciences, Mekdela Amba University, Ethiopia
*Address for Correspondence: Abdu Hussen Ali, Chemistry Department, College of Natural and Computational Sciences, Mekdela Amba University, Ethiopia, Email: [email protected]
Today HPLC is widely applied for separations and purifications in a variety of areas including pharmaceuticals, biotechnology, environmental, polymer and food industries. It is accomplished by injection of a small amount of liquid sample into a moving stream of liquid (called the mobile phase) that passes through a column packed with particles of the stationary phase. The separation of a mixture into its components depends on different degrees of retention of each component in the column. HPLC is just one type of liquid chromatography, meaning the mobile phase is a liquid. Reversed-phase HPLC is the most common type of HPLC. The reversed-phase means the mobile phase is relatively polar, and the stationary phase is relatively non-polar. HPLC instrumentation includes a Solvent reservoir, pump, injector, column, detector, and integrator or acquisition and display system. The heart of the system is the column where separation occurs. The information that can be obtained using HPLC includes identification, quantification, and resolution of a compound. The major applications are in the area of Pharmaceuticals, food, research, manufacturing, forensics, and bio-monitoring of pollutants.
The techniques through which the chemical components present in complex mixtures are Separated, identified, and determined is termed chromatography. This technique is widely used like spectroscopy and is a very powerful tool not only for analytical methods but also for preparative methods. Compounds of high-grade purity can be obtained by this method. Chromatography can be simply defined as follows: “It is the technique in which the components of a mixture are separated based upon the rates at which they are carried or moved through a stationary phase (column) by a gaseous or liquid mobile phase”.
High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography (HPLC) was developed in the late 1960s and early 1970s. Today it is widely applied for separations and purifications in a variety of areas including pharmaceuticals, biotechnology, environmental, polymer and food industries. HPLC has over the past decade become the method of choice for the analysis of a wide variety of compounds. Its main advantage over GC is that the analytes do not have to be volatile, so macromolecules are suitable for HPLC analysis. HPLC is accomplished by injection of a small amount of liquid sample into a moving stream of liquid (called the mobile phase) that passes through a column packed with particles of the stationary phase. The separation of a mixture into its components depends on different degrees of retention of each component in the column. The extent to which a component is retained in the column is determined by its partitioning between the liquid mobile phase and the stationary phase. In HPLC this partitioning is affected by the relative solute/stationary phase and solute/mobile phase interactions. Thus, unlike GC, changes in mobile phase composition can have an enormous impact on your separation. Since the compounds have different mobilities, they exit the column at different times; i.e., they have different retention times, tR. The retention time is the time between injection and detection. Thus, HPLC is most often used when one is performing a target compound analysis, where one has a good idea of the compounds present in a mixture so reference standards can be used for determining retention times [1].
HPLC has gained its quality primarily because of its reliability (use of pressure-driven liquid support) and suppleness (possibility of adjusting the composition of every mobile and stationary phase). The activity mode or separation mechanism depends on the final interactive relationships between the stationary half, the mobile half, and additionally the analyte. Particle-packed columns with either entirely porous- or the newly developed core-shell particles and monolithic columns are utilized in normal or miniaturized HPLC. Quantitative analysis is often accomplished with HPLC. An automatic injector providing reproducible injection volumes is extremely beneficial and is standard on modern commercial systems. HPLCs are rather simple. Good separation of a given pair of compounds by HPLC depends on the choice of column and the efficiency of the overall system.
The relative position of the various components in the sample on the chromatogram is affected by a solute-solvent type of interaction with the column substrate competing with a solute-solvent interaction with the mobile phase. Column efficiency is concerned with the broadening of an initially compact band of solutes as it passes through the column. The broadening is a result of column design and column operating conditions. For samples with a broad range of retention times, it is often desirable to employ solvent programming, whereby the mobile phase composition is varied continuously or in steps as the separation proceeds. The analysis of mixtures of widely varying compositions frequently leads to a very widespread retention time.
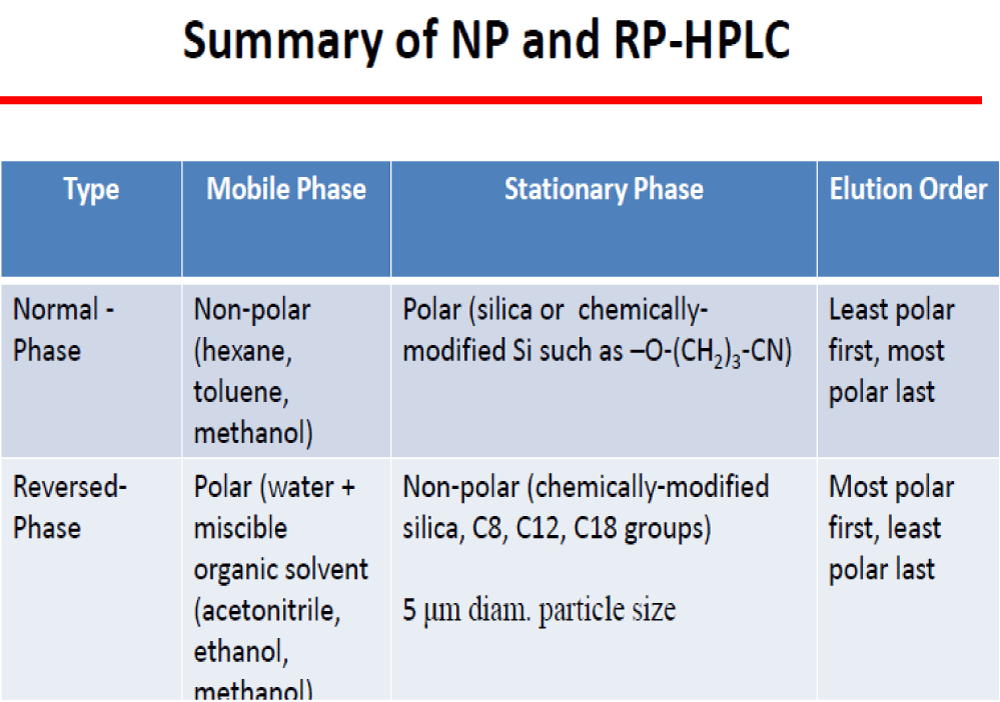
HPLC is just one type of liquid chromatography, meaning the mobile phase is a liquid. Reversed-phase HPLC is the most common type of HPLC. What reversed-phase means is that the mobile phase is relatively polar, and the stationary phase is relatively non-polar. Thus non-polar compounds will be more retained (i.e. have longer retention times) than a polar compounds. In normal phase HPLC, the mobile phase is relatively non-polar and the stationary phase is relatively polar. Other more general types of HPLC include partition, adsorption, ion-exchange, size-exclusion, and thin-layer chromatography.
The goal of the HPLC method is to try, separate, and quantify the main drug, any reaction impurities, all available synthetic intermediates, and any degradants. High-Performance Liquid Chromatography is now one of the most powerful tools in analytical chemistry. It has the ability to separate, identify, and quantify the compounds that are present in any sample that can be dissolved in a liquid. HPLC is the most accurate analytical method widely used for the quantitative as well as qualitative analysis of drug products and used for determining drug product stability [2].
Principle of HPLC
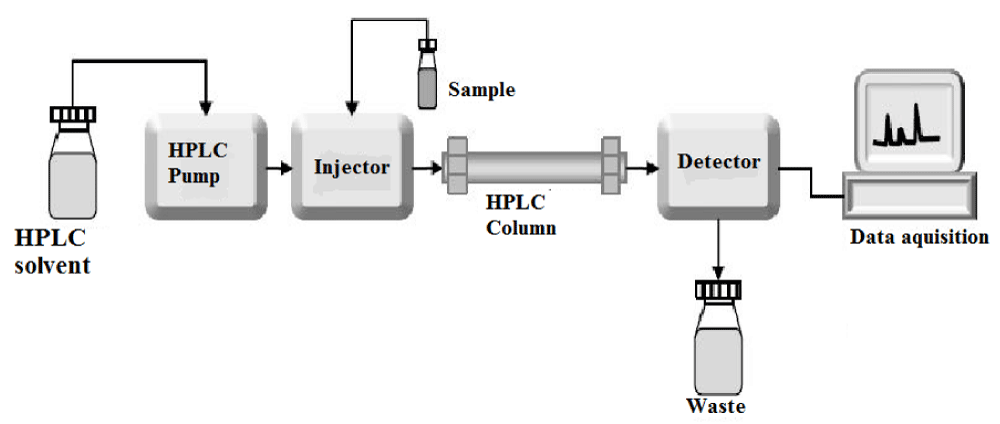
HPLC is a separation technique that involves: The injection of a small volume of liquid sample into a tube packed with tiny particles (3 to 5 micron (μm) in diameter called the stationary phase) where individual components of the sample are moved down the packed tube (column) with a liquid (mobile phase) forced through the column by high pressure delivered by a pump. These components are separated from one another by the column packing that involves various chemical and/or physical interactions between their molecules and the packing particles. These separated components are detected at the exit of this tube (column) by a flow-through device (detector) that measures their amount. Output from this detector is called an “HPLC” In principle, LC and HPLC work the same way except for the speed, efficiency, sensitivity, and ease of operation of HPLC are vastly superior. Though HPLC retains major of the credits for the analytical side, the earlier one of simple Liquid Chromatography still finds applications for the preparative purposes Figure 1.
Figure 1: Component of HPLC.
As shown in the schematic diagram in the figure above, HPLC instrumentation includes a Solvent reservoir, pump, injector, column, detector, and integrator or acquisition and display system. The heart of the system is the column where separation occurs.
Solvent reservoir
Mobile phase contents are contained in a glass reservoir. The mobile phase, or solvent, in HPLC, is usually a mixture of polar and non-polar liquid components whose respective concentrations are varied depending on the composition of the sample. The type and composition of the mobile phase affect the separation of the components. For HPLC we use high-grade solvent. Different solvents are used for different types of HPLC. For normal-phase HPLC, the solvent is usually non-polar, and, in reverse-phase HPLC, the solvent is normally a mixture of water and a polar organic solvent. The purity of solvents and inorganic salts used to make the mobile phase is paramount [3].
The most common solvent reservoirs are as simple as glass bottles with tubing connecting them to the pump inlet.
✓ vacuum pumping system ✓ distillation system ✓ devices for heating and stirring ✓ System for sparging, in which the dissolved gases are swept out of solution by fine bubbles of an inert gas that is not soluble in the mobile phase. ✓ Often the systems also contain a means of filtering dust and particulate matter from the solvents to prevent these particles from damaging the pumping or injection Systems or clogging the column. It is not necessary that the degassers and filters be integral parts of the HPLC system. Elution with a single solvent or solvent mixture of constant composition is termed an isocratic elution. In gradient elution, two (and sometimes more) solvent systems that differ significantly in polarity are used and varied in composition during the separation. A pump can be compared to the human heart which continuously pumps blood throughout the body but through the human heart can withstand changes in blood pressure within a specified limit due to stress and strain the HPLC pump is required to deliver a flow of mobile phase at constant pressure and flow rate. Changes in both these parameters can lead to errors in the results. In simple language, the HPLC pump has to have ruggedness and at the same time should be able to provide reproducible flow characteristics run after run. The operational pressure limits have a vast range depending upon analysis requirements. In normal analytical operation the pressure can vary between 2000 – 5000 psi but in applications covered under UHPLC mode operating pressure can be as high as 15000 – 18000 psi. All HPLC systems include at least one pump to force the mobile phase through whose packing is fairly compact. The result of this is a pressure increase at the injector which can attain 20 000 kPa (200 bars) depending upon the flow rate imposed upon the mobile phase, its viscosity, and the size of the particles of the stationary phase. Pumps are designed in order to maintain a stable flow rate, avoiding pulsations even when the composition of the mobile phase varies. These flow rate metered pumps contain, in general, two pistons in series, working in opposition, to avoid interruptions to the flow rate [4]. A pump aspirates the mobile phase from the solvent reservoir and forces it through the system’s column and detector. Depending on a number of factors including column dimensions, the particle size of the stationary phase, the flow rate and composition of the mobile phase, operating pressures of up to 42000 kPa (about 6000 psi) can be generated. The role of the pump is to force a liquid (called the mobile phase) through the liquid chromatography at a specific flow rate, expressed in milliliters per min (mL/min). Normal flow rates in HPLC are in the 1-to 2-mL/min range. Typical pumps can reach pressures in the range of 6000-9000 psi (400-to 600-bar). During the chromatographic experiment, a pump can deliver a constant mobile phase composition (isocratic) or an increasing mobile phase composition (gradient) variations in flow rates of the mobile phase affect the elution time of sample components and result in errors. Pumps provide a constant flow of mobile phase to the column under constant pressure. An ideal pump should have the following desirable characteristics: • Solvent compatibility and resistance to corrosion • Constant flow delivery independent of back pressure • Convenience of replacement of worn-out parts • Low dead volume for minimum problems on solvent change over ♦ Three commonly used pump types are Syringe type pumps, Constant pressure pumps, and Reciprocating piston pumps. Constant pressure pumps: This provides a consistent continuous flow rate through the column with the use of pressure from a gas cylinder. Valving arrangement allows rapid refill of the solvent chamber. A low-pressure gas source is needed to generate high liquid pressures. Syringe type pumps: It is suitable for small bore columns. The constant flow rate is delivered to the column by a motorized screw arrangement. The solvent delivery rate is set by changing the voltage on the motor. These pumps deliver pulse less flow independent of column back pressure and changes in viscosity but major disadvantages are limited solvent capacity and limitation on gradient operation. Reciprocating piston pumps: These deliver solvent(s) through the reciprocating motion of a piston in a hydraulic chamber. On the backstroke, the solvent is sucked in and gets delivered to the column in the forward stroke. Flow rates can be set by adjusting piston displacement in each stroke. Dual and triple head pistons consist of identical piston chamber units which operate at 1800 or 1200 phase differences. The solvent delivery of reciprocating pump systems is smooth because while one pump is in the filling cycle the other is in the delivery cycle. High-pressure output is possible at a constant flow rate and gradient operation is possible. However, pulse dampening is required for further elimination of pressure pulses. The injector can be a single injection or an automated injection system. An injector for an HPLC system should provide an injection of the liquid sample within the range of 0.1-100 mL of volume with high reproducibility and under high pressure (up to 4000 psi). The injector must also be able to withstand the high pressures of the liquid system. An autosampler is an automatic version for when the user has many samples to analyze or when the manual injection is not practical. Injectors are used to provide constant volume injection of samples into the mobile phase stream. Inertness and reproducibility of injection are necessary to maintain a high level of accuracy [5]. In HPLC, the injection of a precise volume of sample onto the head of the column must be made as fast as possible in order to cause the minimum disturbance to the dynamic regime of the mobile phase whose flow must be stable from column to detector. This is done by a special high-pressure valve, either manual or motorized, possessing several flow paths, which is situated just prior to the column. This must be a component of precision able to resist pressures greater than 30 000 kPa. The valve functions in two positions: • In the load position only communication between the pump and the column is assured. The sample, contained in a solution, is introduced at atmospheric pressure with the aid of a syringe into a small tubular curved section named a loop. Each loop has a small defined volume. They are either integrated into the rotor of the valve or are connected to the outside of the valve’s casing. • In the inject position, the sample (which is in the loop) is inserted into the flow of the mobile phase by the 60_ rotation of a part of the valve, thus connecting the sample loop to the mobile phase circulation. Highly reproducible injections are attained only if the loop has been completely filled with the sample. Injectors serve to introduce the required sample volume accurately into the HPLC system. Sample injection into the moving mobile phase stream in HPLC is quite different from injection into a gas stream in Gas Chromatography as the precise injection is required against high back pressure. In such a situation it is not possible to simply inject using a syringe alone. The type of injector is: Manual injection (Rheodyne/Valco injectors): The injection is done through a specially designed 6-port rotary injection valve. The sample is introduced at atmospheric pressure by a syringe into a constant volume loop. In the LOAD position, the loop is not in the path of the mobile phase. By rotating to the INJECT position the sample in the loop is moved by the mobile phase stream into the column. It is important to allow some samples to flow into waste from the loop so as to ensure there are no air bubbles in the loop and the previously used sample is completely washed out to prevent memory effects. Automatic injection: Automatic injection improves laboratory productivity and also eliminates personal errors. Present-day advanced HPLC systems are equipped with an auto-injector along with an auto-sampler. The software programs filling of the loop and delivery the sample to the column. The computer also controls the sequence of samples for injection from vials kept in numbered positions of the autosampler. It is important to adopt precautions to ensure consistency of results and also prolong the service life of the automated system. Prime injector with solvents to be used but it should be ensured that solvent is compatible with the solvent used earlier. Needle wash between samples will prevent carry-over between injections. Before the start and at end of analysis ensure tubing is completely washed of buffers or previously used solvents. Do not forget to feed the vial number correctly on the autosampler rack and list out the Sequence correctly on the computer [6]. Columns are usually made of polished stainless steel, are between 50 and 300 mm long, and have an internal diameter of between 2 and 5 mm. They are commonly filled with a stationary phase with a particle size of 3–10 µm. Columns with internal diameters of less than 2 mm are often referred to as microbore columns. Ideally, the temperature of the mobile phase and the column should be kept constant during an analysis. Considered the “heart of the chromatograph” the column’s stationary phase separates the sample components of interest using various physical and chemical parameters. The small particles inside the column are what causes the high back pressure at normal flow rates. The pump must push hard to move the mobile phase through the column and this resistance causes a high pressure within the chromatograph [7]. It is a vital component and should be maintained properly as per supplier instructions for getting reproducibility separation efficiency run after run. The types of columns are: Guard columns: A guard column is introduced before the analytical column to increase the life of the analytical column by removing not only particulate matter and contaminants from the solvents but also sample components that bind irreversibly to the stationary phase. The guard column serves to saturate the mobile phase with the stationary phase so that losses of this solvent from the analytical column are minimized. The composition of the guard-column packing is similar to that of the analytical column; the particle size is usually larger. When the guard column has become contaminated, it is repacked or discarded and replaced with a new one. Analytical columns: It is the heart of High-performance liquid chromatography. Liquid-chromatographic columns range in length from 10 to 30 cm. normally, the columns are straight, with added length, where needed, being gained by coupling two or more columns together. The inside diameter of liquid columns is often 4 to 10 mm; the most common particle size of packing is 5 or 10 m. The most common column currently in use is one that is 25 cm in length, 4.6 mm inside diameter, and packed with 5 mm particles. Columns of this type contain 40,000 to 60,000 plates/meter Figure 2.
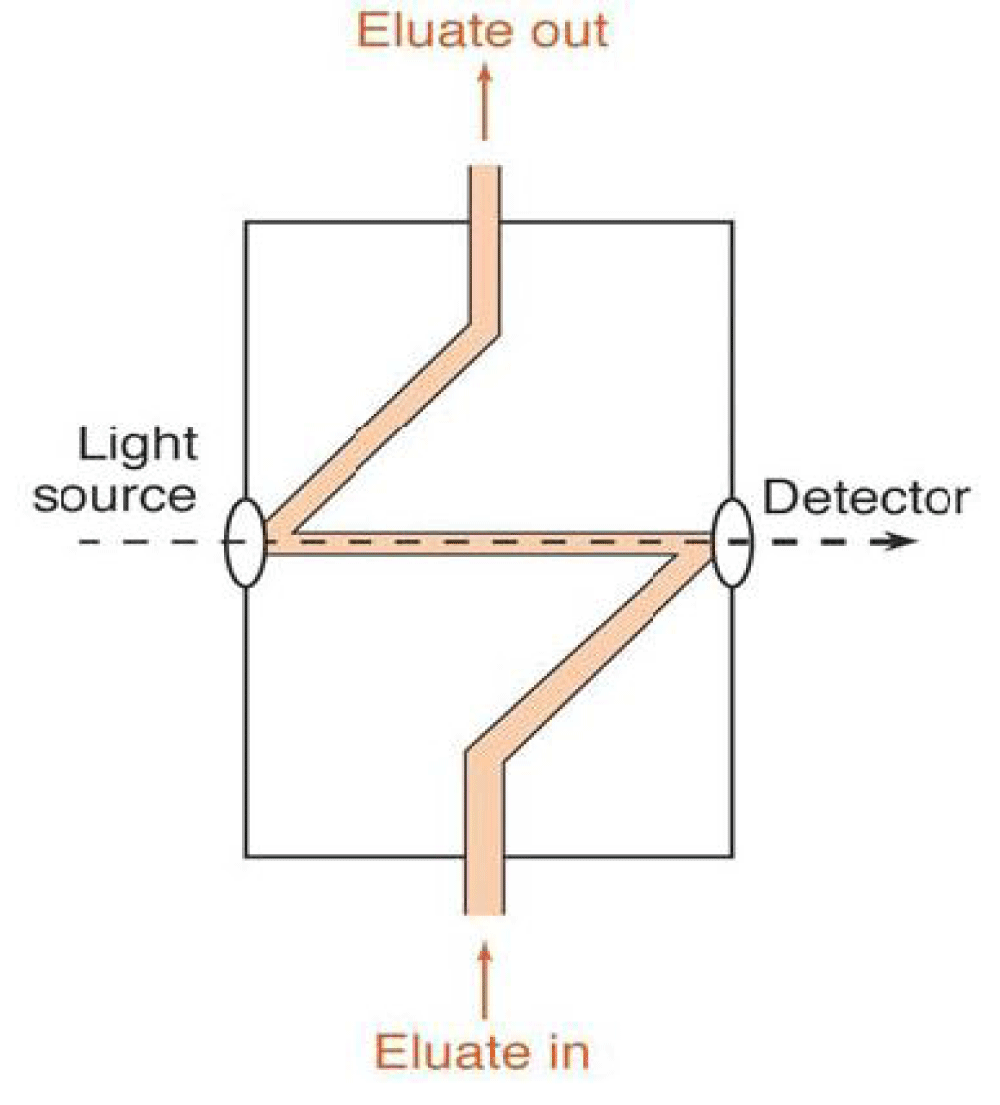
Figure 2: Typical HPLC column. HPLC columns are mostly made up of smooth bore stainless steel. HPLC columns are sometimes made from heavy-walled glass tubing and polymer tubing, such as polyether ether ketone. Column temperature control: For some applications, close control of column temperature is not necessary and columns are operated at room temperature. Often, however, better, more reproducible chromatograms are obtained by maintaining constant column temperature. Three ways the temperature of a column could be controlled are oven, heater block, and water bath. But now a day’s modern instruments may need a higher temperature with limitations [8]. The HPLC detector, located at the end of the column detects the analytes as they elute from the chromatographic column. Commonly used detectors are evaporative light scattering, UV-spectroscopy, fluorescence, mass-spectrometric and electrochemical detectors. The detector can see (detect) the individual molecules that come out (elute) from the column. A detector serves to measure the amount of those molecules so that the chemist can quantitatively analyze the sample components. The detector provides an output to a recorder or computer that results in the liquid chromatogram (i.e., the graph of the detector response). A detector gives a specific response for the components separated by the column and also provides the required sensitivity. It has to be independent of any changes in mobile phase composition. Monitoring the mobile phase as it emerges from the column [9]. The ideal characteristics ✓ Adequate sensitivity for the particular task. ✓ Good stability and reproducibility. ✓ The wide linear dynamic range of response. ✓ Short response time that is independent of flow rate. ✓ Insensitive to changes in a solvent, flow rate, and temperature. ✓ Cell design that eliminates remixing of the separated bands. ✓ High reliability and ease of use. ✓ Non-destructive for the sample. Evaporative light scattering detectors (ELSDs): ELSD is ideal for detecting analytes with no UV chromophore as they do not rely on the optical properties of a compound. ➢ High sensitivity provides superb responses for all compounds, down to low nanogram levels. ➢ Real-time control during an injection using pro-grammable Dimension Software maintains maximum sensitivity throughout the run. ➢ Real-time gas programming using Dimension Software eliminates solvent enhancement effects during gradient elution, for excellent quantification. ➢ Low dispersion and high-speed data output rates are the perfect match for fast LC applications. ➢ Superb reproducibility below 2% gives reliable and accurate results ➢ Multivendor software control and data acquisition using Agilent ChemStation chromatography data system, and other vendors’ interfaces, eliminates the need for an analog to digital converter. ➢ Rapid heating and cooling of the evaporator tube minimize equilibration time. ➢ Full DMSO transparency ensures that responses from early eluting compounds are not hidden. ➢ Fully integrated with all Agilent analytical and preparative LC systems for the complete chromato-graphic solution. ➢ Complementary to LC/MS. Refractive index detectors ✓ The nearly universal but poor detection limit ✓ Passes visible light through 2 compartments, sample &reference. ✓ When the solvent composition is the same the light passed through the compartments the light beam that passes through is recorded as zero. ✓ When a solute is in the sample compartment, refractive index changes will shift the light beam from the detector. ✓ Limit of detection (LOD) 10 ng of solute. U.V detectors • Based on electronic transitions within molecules. • A most common type of detector for LC • The fixed wavelength, Hg lamp 254 nm (π = > π*) • The tunable wavelength is selectable for specific wavelengths, monochromators, or filters Figure 3. • Still limited to single wavelengths. • Solvent limitations with UV-vis abs. Detectors • Z-shape, flow-through cell (V, 1 ~ 10 μL and b, 2 ~ 10 mm) • Spectrophotometer: more versatile.
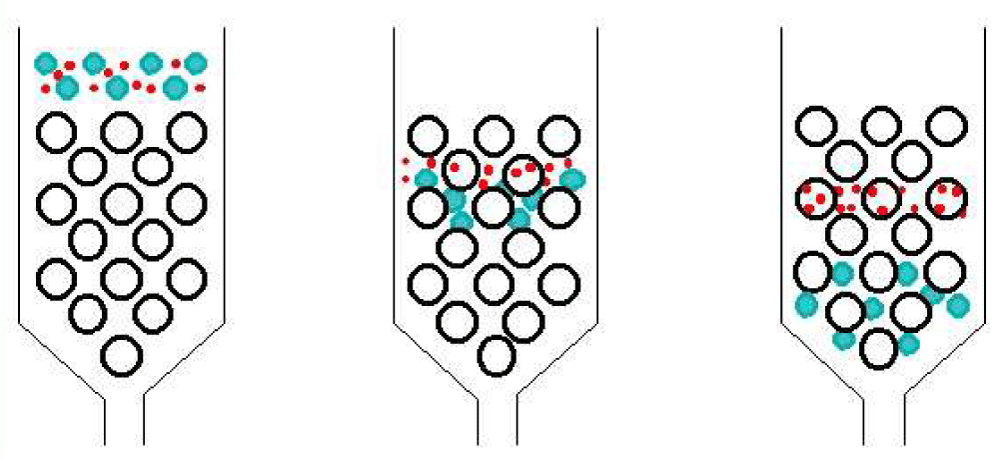
Figure 3: UV detector. Fluorescence detectors • Fluorescence rays emitted by the sample after absorbing incident light are measured. • A xenon arc lamp is used to produce light for excitation. • Only suitable for compounds that can produce fluorescence. • High precision and sensitivity. • Some compounds are not stable under fluorescent stimulation. Electrochemical detectors • Based on the amperometric response of analyte to electrode usually held at a constant potential. • If the analyte is electro-active, can be highly sensitive since the response is based on a surface phenomenon rather than a solution bulk property (e.g. UV-vis absorbance) • simplicity, convenience, and wide-spreading application • The thin-layer flow cell of Teflon: 50μm thick, 1 ~ 5 μL volume • Indictor E: Pt, Au, C • Multi-electrode: simultaneous detection or sample purity indication. Mass spectroscopy detector • Universal detector • An MS detector senses a compound eluting from the HPLC column first by ionizing it then by measuring its mass and/or fragmenting the molecule into smaller pieces that are unique to the compound. • The MS detector can sometimes identify the compound directly since its mass spectrum is like a fingerprint and is unique to that compound. IR detectors • Filter instrument or FTIR • Similar cell (V, 1.5 ~ 10 μL and b, 0.2 ~ 1.0 mm) • Limit: no suitable solvent, special optics • FT-IR allows for spectrum records of flowing systems analogous to the diode array system. • Water/alcohols can be major interferences to solute detection • LOD 100 ng (limit of detection) Signals from the detector may be collected on chart recorders or electronic integrators that vary in complexity and in their ability to process, store and re-process chromatographic data. The computer integrates the response of the detector to each component and places it into a chromatograph that is easy to read and interpret. Frequently called the data system, the computer not only controls all the modules of the HPLC instrument but it takes the signal from the detector and uses it to determine the time of elution (retention time) of the sample components (qualitative analysis) and the amount of sample (quantitative analysis) [10]. Samples in liquid form can be analyzed directly, after a suitable clean-up to remove any particulate materials or after a suitable extraction to remove matrix interferents. In determining polyaromatic hydrocarbons (PAH) in wastewater, for example, an initial extraction with CH2Cl2 serves the dual purpose of concentrating the analytes and isolating them from matrix interferents. Solid samples must first be dissolved in a suitable solvent, or the analytes of interest must be brought into solution by extraction. For example, an HPLC analysis for the active ingredients and degradation products in a pharmaceutical tablet often begins by extracting the powdered tablet with a portion of the mobile phase. Gases are collected by bubbling through a trap containing a suitable solvent. Organic isocyanates in industrial atmospheres can be determined in this manner by bubbling the air through a solution of 1-(2-methoxyphenyl) piperazine in toluene. Reacting the isocyanates with 1-(2-methoxyphenyl) piperazine serves the dual purpose of stabilizing them against degradation before the HPLC analysis while also forming a derivative that can be monitored by UV absorption [11]. A. Based on modes of chromatography 1. Normal–Phase chromatography: Normal–phase chromatography was one of the first kinds of HPLC that chemists developed. Also known as normal-phase HPLC (NP-HPLC) this method separates analytes based on their affinity for a polar stationary surface such as silica; hence it is based on the analyte’s ability to engage in polar interactions (such as hydrogen-bonding or dipole-dipole type of interactions) with the sorbent surface. NP-HPLC uses a non-polar, non-aqueous mobile phase (e.g. Chloroform, Octane), and works effectively for separating analytes readily soluble in non-polar solvents. The analyte associates with and is retained by the polar stationary phase [12]. 2. Hydrophilic interaction chromatography: Hydrophilic interaction chromatography (HILIC) can be described as a reversed reversed-phase chromatography performed using a polar stationary phase (for example, unmodified silica, amino, or diol bonded phases). The mobile phase employed is highly organic in nature (>70% solvent, typically acetonitrile) containing also a small percentage of aqueous solvent/buffer or another polar solvent. The water/polar solvent forms an aqueous-rich sub-layer adsorbed to the polar surface of the stationary phase into which analytes partition. The retention mechanisms in HILIC are complex but are believed to be a combination of hydrophilic partitioning interaction and secondary electrostatic and hydrogen-bonding interactions. These mechanisms result in an elution order that is roughly the opposite of that in the reversed-phase. Although the organic modifier/aqueous ratio is the predominant factor in providing the necessary separation selectivity in HILIC, the choice of stationary phase is also important in matching the column chemistry to the analyte functional groups. 3. Reversed-Phase Chromatography (RPC): Reversed-phase HPLC (RP-HPLC) has a non-polar stationary phase and an aqueous, moderately polar mobile phase. One common stationary phase is silica which has been surface-modified with RMe2SiCl, where R is a straight-chain alkyl group such as C18H37 or C8H17. With such stationary phases, retention time is longer for molecules that are less polar, while polar molecules elute more readily (early in the analysis). An investigator can increase retention times by adding more water to the mobile phase; thereby making the affinity of the hydrophobic analyte for the hydrophobic stationary phase stronger relative to the now more hydrophilic mobile phase. Similarly, an investigator can decrease retention time by adding a more organic solvent to the eluent [13]. B. Based on the principle of separation Ion exchange chromatography: In ion-exchange chromatography (IC), retention is based on the attraction between solute ions and charged sites bound to the stationary phase. Solute ions of the same charge as the charged sites on the column are excluded from binding, while solute ions of the opposite charge of the charged sites of the column are retained on the column. Solute ions that are retained on the column can be eluted from the column by changing the solvent conditions (e.g. increasing the ion effect of the solvent system by increasing the salt concentration of the solution, increasing the column temperature, changing the pH of the solvent, etc...). In general, ion exchangers favor the binding of ions of higher charge and smaller radii. An increase in counter ion (concerning the functional groups in resins) concentration reduces the retention time. A decrease in pH reduces the retention time in cation exchange while an increase in pH reduces the retention time in anion exchange. By lowering the pH of the solvent in a cation exchange column, for instance, more hydrogen ions are available to compete for positions on the anionic stationary phase, thereby eluting weakly bound cations [14]. This form of chromatography is widely used in the following applications: water purification, preconcentration of trace components, ligand-exchange chromatography, ion-exchange chromatography of proteins, high-pH anion-exchange chroma-tography of carbohydrates, and oligosaccharides, and others. The technique is well suited for: ✓ The separation of inorganic and organic anions and cations is an aqueous solution. ✓ Ionic dyes, amino acids, and proteins can be separated by ion exchange because such compounds are salt in brine water, It is also useful for determining the tertiary structure and quaternary structure of purified proteins. SEC is used primarily for the analysis of large molecules such as proteins or polymers. SEC works by trapping these smaller molecules in the pores of a particle. The larger molecules simply pass by the pores as they are too large to enter the pores. Larger molecules, therefore, flow through the column quicker than smaller molecules, that is, the smaller the molecule, the longer the retention time. This technique is widely used for the molecular weight determination of polysaccharides. In SEC, there is no interaction between the sample compounds and the column packing material. Instead, molecules diffuse into the pores of a porous medium. Depending on their size relative to the pore size, molecules are separated. Molecules larger than the pore opening do not diffuse into the particles, while molecules smaller than the pore opening enter the particle and are separated. Large molecules elute first. Smaller molecules elute later [15]. It is classified into two categories based on the nature of the columns and their packing i. Gel Filtration Chromatography: Which uses hydrophilic packing to separate polar species and uses mostly aqueous mobile phases. This technique is mostly used to identify the molecular weights of large-sized proteins & bio-molecules. ii. Gel Permeation Chromatography: Which uses hydrophobic packing to separate non-polar species and uses non-polar organic solvents. This technique is used to identify the molecular weights of polymers Figure 4.
Figure 4: SEC separation Affinity chromatography Affinity chromatography involves covalently bonding a reagent called an affinity ligand, to a solid support. Typical affinity ligands are antibodies, enzyme inhibitors, cofactor/coenzyme, or other molecules that reversibly and selectively bind to analyte molecules in the sample. The principle is that the stationary phase consists of a support medium (e.g. cellulose beads) on which the substrate (or sometimes a coenzyme) has been bound covalently, in such a way that the reactive groups that are essential for enzyme binding are exposed. As the mixture of proteins is passed through the chromatography column, those proteins that have a binding site for the immobilized substrate will bind to the stationary phase, while all otter proteins will be eluted in the void volume of the column. When the sample passes through the column, only the molecules that selectively bind to the affinity ligand are retained. Molecules that don’t bind pass through the column with the mobile phase. After the undesired molecules are removed, the retained analytes can be eluted by changing the mobile-phase conditions. Once they bonded themselves later, they must be separated from bonded stationary phase using another solvent which has a good capacity for separation. Mostly it is useful for the separation of biomolecules like protein [16]. Internal diameter: The internal diameter (ID) of an HPLC column is a critical aspect that determines the quantity of analyte that can be loaded onto the column and also influences sensitivity. Larger columns are usually seen in industrial applications such as the purification of a drug product for later use. Low ID columns have improved sensitivity and lower solvent consumption at the expense of loading capacity. Particle size: Most traditional HPLC is performed with the stationary phase attached to the outside of small spherical silica particles (very small beads). Smaller particles generally provide more surface area and better separations, but the pressure required for optimum linear velocity increases by the inverse of the particle diameter squared. Pore size: Many stationary phases are porous to provide greater surface area. Small pores provide greater surface area while larger pore size has better kinetics, especially for larger analytes. Pore size defines the ability of the analyte molecules to penetrate inside the particle and interact with its inner surface. This is especially important because the ratio of the outer particle surface to its inner one is about 1:1000. The surface molecular interaction mainly occurs on the inner particle surface. Pump pressure: Pumps vary in pressure capacity, but their performance is measured by their ability to yield a consistent and reproducible flow rate. Modern HPLC systems have been improved to work at much higher pressures, and therefore be able to use much smaller particle sizes in the columns Temperature: For proper function of the HPLC the temperature has its own influence. Mostly HPLC columns can work at room temperature or around (25-35 ◦c) are good. But there is also an unexceptional case that requires a higher temperature. For the accurate analysis of a compound, there are some parameters that are used as a standard for a particular compound. If there is a change occurs in the parameters the result may be affected greatly. The most commonly used parameters are internal diameter, particle size, pore size, and pump pressure. For different compounds, the parameters can be changed according to their nature and chemical properties. 1. Retention time: Retention time is the difference in time between the point of injection and the appearance of peak maxima. It is also defined as the time required for 50% of a component to be eluted from a column. It is measured in minutes and seconds. 2. Retention volume: Retention volume is the volume of carrier gas required to elute 50% of the component from the column. It is the product of retention time and flow rate. Retention volume = Retention time × flow rate 3. Separation factor: Separation factor is the ratio of partition coefficient of the 2 components to be separated. S= Ka/Kb = (tb-to) / (ta-to) Where to = Retention time of unretained substance. Ka, Kb = Partition coefficients of a, b ta, tb = Retention time of substance a, b If there is a more difference in partition coefficient between 2 compounds, the peaks are far apart and the separation factor is more. If the partition coefficients of the 2 compounds are similar, then the peaks are closer and the separation factor is less. 4. Resolution: Resolution is the measure of the extent of separation of 2 components and the baseline separation achieved. Rs = 2 (Rt1-Rt2) / w1+w2 5. Height Equivalent to a Theoretical Plate (HETP): A theoretical plate is an imaginary or hypothetical unit of a column where the distribution of a solute between the stationary phase and mobile phase has attained equilibrium. It can also be called a functional unit of the column. A theoretical plate can be of any height, which describes the efficiency of separation. If HETP is less, the column is more efficient. 6. Efficiency: The efficiency of a column is expressed by the theoretical plates. n = 16 Rt2/ w2 Where n = no of theoretical plates Rt = retention time w = peak width at base. 7. Asymmetry factor: A chromatographic peak should be symmetrical about its center. But in practice due to some factors, the peak is not symmetrical and shows tailing or fronting. Fronting is due to saturation of stationary phase and can be avoided by using less quantity of sample. Tailing is due to more active adsorption sites and can be eliminated by support pretreatment. Asymmetry factor (0.95 to 1.05) can be calculated by AF = b/a (b, a calculated by 5% or 10% of the peak height). Broad peaks occur due to the more conc. of the sample, large injection volume, and column deterioration. Advantages ✓ Separations are fast and efficient (high-resolution power) ✓ Continuous monitoring of the column effluent ✓ It can be applied to the separation and analysis of very complex mixtures ✓ Accurate quantitative measurements. ✓ Repetitive and reproducible analysis using the same column. ✓ Adsorption, partition, ion exchange, and exclusion column separations are excellently made. ✓ HPLC is more versatile than GLC in some respects because it has the advantage of not being restricted to volatile and thermally stable solute and the choice of mobile and stationary phases is much wider in HPLC. ✓ Aqueous and non-aqueous samples can be analyzed with little or no sample pre-treatment. ✓ A variety of solvents and column packing are available, providing a high degree of selectivity for specific analyses. ✓ It provides a means for the determination of multiple components in a single analysis and etc. Disadvantages ✓ Column performance is very sensitive, which depends on the method of Packing. ✓ Further, no universal and sensitive detection system is available. ✓ Very costive, have low sensitivity for certain compounds, and some cannot be detected as they are irreversibly adsorbed. The information that can be obtained using HPLC includes the identification, quantification, and resolution of a compound. Preparative HPLC refers to the process of isolation and purification of compounds. This differs from analytical HPLC, where the focus is to obtain information about the sample compound. The major applications are the following. High-Performance Liquid Chromatography provides reliable quantitative precision and accuracy along with a high linear dynamic range to allow the determination of API and related substances in a single run. A convenient method for sample preparation for solid dosage forms is dispersion in water or aqueous media modified with acetonitrile or methanol. HPLC offers several possibilities for the separation of chiral molecules into their respective enantiomers. These include precolumn derivatization to form diastereomers. Alternately, specialty columns prepared with cyclodextrins or special chiral moieties as stationary phases may be used. In short HPLC, particularly reverse phase HPLC is the most popular choice for quantitative analysis in the pharmaceutical industry. Common application areas in the pharmaceutical analysis are: • To control drug stability. • Tablet dissolution study of the pharmaceutical dosage form. • Pharmaceutical quality control. • Assay • Related Substances • Analytical Method Validation • Stability Studies • Compound Identification • Working Standards. High-Performance Liquid Chromatography has brought desirable advantages in the field of food analysis. Food matrices are generally complex and extraction of analytes is not an easy task. To further complicate matters both desirable and undesirable components are often found in trace levels and classical extraction and analysis do not provide the required levels of accuracy and precision. HPLC offers viable solutions due to the vast choice of stationary phases and mobile phase options. ∗ Common applications in foods are • Fat-soluble vitamins (A, D, E, and K) • Water-soluble vitamins (B-complex vitamins such as B1, B2, B3, B6, Folic acid, Pantothenic acid, B12, Vitamin C) • Residual pesticides such as 2, 4-D, and Mono-chrotophos. • Antioxidants such as TBHQ, BHA, and BHT. • Sugars: Glucose, Fructose, Maltose, and other saccharides • Cholesterol and sterols • Mycotoxins such as Aflatoxins B1, B2, G1, G2, M1, M2, and ochratoxin • Amino acids • Residual antibiotics • Steroids and flavanoids • Aspartame and other artificial sweeteners. HPLC has many applications in both laboratory and clinical science. It is a common technique used in pharmaceutical development as it is a dependable way to obtain and ensure product purity. While HPLC can produce extremely high-quality (pure) products, it is not always the primary method used in the production of bulk drug materials. According to the European pharmacopeia, HPLC is used in only 15.5% of syntheses. However, it plays a role in 44% of syntheses in the United States pharmacopeia. This could be due to differences in monetary and time constraints, as HPLC on a large scale can be an expensive technique. An increase in specificity, precision, and accuracy that occurs with HPLC, unfortunately, corresponds to an increase in cost. Similar assays can be performed for research purposes, detecting concentrations of potential clinical candidates like anti-fungal and asthma drugs. This technique is obviously useful in observing multiple species in collected samples, as well, but requires the use of standard solutions when information about species identity is sought out. It is used as a method to confirm the results of synthesis reactions, as purity is essential in this type of research. Medical use of HPLC can include drug analysis but falls more closely under the category of nutrient analysis. While urine is the most common medium for analyzing drug concentrations, blood serum is the sample collected for most medical analyses with HPLC. Other methods of detection of molecules that are useful for clinical studies have been tested against HPLC, namely immunoassays. In one example of this, competitive protein binding assays (CPBA) and HPLC were compared for sensitivity in the detection of vitamin D. Useful for diagnosing vitamin D deficiencies in children, it was found that sensitivity and specificity of this CPBA reached only 40%, and 60%, respectively, of the capacity of HPLC. While an expensive tool, the accuracy of HPLC is nearly unparalleled. Other application of HPLC includes • Environmental Applications 1. Detection of phenolic compounds in drinking water. 2. Bio-monitoring of pollutants. • Applications in Forensics 1. Quantification of drugs in biological samples. 2. Identification of steroids in blood, urine, etc. 3. Forensic analysis of textile dyes. 4. Determination of cocaine and other drugs of abuse in blood, urine, etc. • Food and Flavour 1. Measurement of Quality of soft drinks and water. 2. Sugar analysis in fruit juices. 3. Analysis of polycyclic compounds in vegetables. 4. Preservative analysis. • Applications in Clinical Tests 1. Urine analysis, antibiotics analysis in blood. 2. Analysis of bilirubin, biliverdin in hepatic disorders. 3. Detection of endogenous neuropeptides in the extracellular fluid of the brain, etc. HPLC is optimum for the separation of chemical and biological compounds that are non-volatile. Typical non-volatile compounds are: ✓ Pharmaceuticals like aspirin, ibuprofen, or acetaminophen (Tylenol) ✓ Salts like sodium chloride and potassium phosphate ✓ Proteins like egg white or blood protein ✓ Organic chemicals like polymers (e.g. polystyrene, polyethylene) ✓ Heavy hydrocarbons like asphalt or motor oil ✓ Many natural products such as ginseng, herbal medicines, plant extracts ✓ Thermally unstable compounds such as trinitrotoluene (TNT), enzymes. Pump
Sample injector
Columns
Detector
Type of detector
Data collection devices (Computer)
Sample preparation for HPLC
Clasification of HPLC
Size Exclusion Chromatography (SEC)
The factors which influence the HPLC performance
Parameter used in HPLC
Advantage and disadvantages of HPLC
Applications of High-Performance Liquid Chromato-graphy (HPLC)
Pharmaceuticals
Foods
Manufacturing
Research
Medical
High-Performance Liquid Chromatography (HPLC)analysis
HPLC method is important to separate and quantify the main drug and any reaction impurities. In HPLC, the mobile phase is a liquid. Reversed-phase HPLC is the most common type of HPLC. What reversed-phase means is that the mobile phase is relatively polar, and the stationary phase is relatively non-polar. Therefore, the non-polar compounds will be more retained (i.e. have longer retention times) than a polar compound. In normal phase HPLC, the mobile phase is relatively non-polar and the stationary phase is relatively polar. These components are separated from one another by the column packing that involves various chemical and/or physical interactions between their molecules and the packing particles. These separated components are detected at the exit of a column by a flow-through device (detector) that measures their amount. Output from this detector is called an “HPLC” in principle, LC and HPLC work the same way except for the speed, efficiency, sensitivity, and ease of operation of HPLC are vastly superior. It is also the most accurate analytical method widely used for the quantitative as well as qualitative analysis of drug products and used for determining drug product stability.
- Gerber F, Krummen M, Potgeter H, Roth A, Siffrin C, Spoendlin C. Practical aspects of fast reversed-phase high-performance liquid chromatography using 3 microm particle packed columns and monolithic columns in pharmaceutical development and production working under current good manufacturing practice. J Chromatogr A. 2004 May 21;1036(2):127-33. doi: 10.1016/j.chroma.2004.02.056. PMID: 15146913.
- Xiang Y, Liu Y, Lee ML. Ultrahigh pressure liquid chromatography using elevated temperature. J Chromatogr A. 2006 Feb 3;1104(1-2):198-202. doi: 10.1016/j.chroma.2005.11.118. PMID: 16376355.
- Bergh JJ, Breytenbach JC. Stability-indicating high-performance liquid chromatographic analysis of trimethoprim in pharmaceuticals. J Chromatogr. 1987 Jan 30;387:528-31. doi: 10.1016/s0021-9673(01)94565-0. PMID: 3558640.
- Haginaka J, Yasuda H, Uno T, Nkagawa T. Alkaline degradation and determination by high-performance by high-performance liquid chromatography. Chemical Pharmacy. Bullet. 1984; 32: 2752-2758
- Fredj G, Paillet M, Aussel F, Brouard A, Barreteau H, Divine C, Micaud M. Determination of sulbactam in biological fluids by high-performance liquid chromatography. J Chromatogr. 1986 Nov 28;383(1):218-22. doi: 10.1016/s0378-4347(00)83464-7. PMID: 3029153.
- Ayrton J. Assay of ceftazidime in biological fluids using high-pressure liquid chromatography. J Antimicrob Chemother. 1981 Sep;8 Suppl B:227-31. doi: 10.1093/jac/8.suppl_b.227. PMID: 19802990.
- Polite L. Liquid chromatography: basic overview. In: Miller J, Crowther JB [eds], Analytical chemistry in a GMP environment: a practical guide. John wiley & sons, New York. 2000.
- United States Pharmacopoeia. 27th ed. The USP Convention Inc., Rockville, MD. 2014.
- Rodenas V, Garcia MS, Sanchez-Pedreno C, Albero MI. Flow-injection spectrophotometric determination of frusemide or sulphathiazole in pharmaceuticals. Journal of Pharmacy and Biomedical Analyst. 1997; 15: 1687-1693.
- Chemical Analysis Modern Instrumentation Methods and Techniques, Second Edition, Francis Rouessac and Annick Rouessac, University of Le Mans, France.
- Abidi SL. High-performance liquid chromatography of phosphatidic acids and related polar lipids. Journal of Chromatography. 1991; 587: 193-203.
- The European Pharmacopoeia. fourth ed., Council of Europe, Strasbourg. 2002.
- Tsai IL, Weng TI, Tseng YJ, Tan HK, Sun HJ, Kuo CH. Screening and confirmation of 62 drugs of abuse and metabolites in urine by ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry. J Anal Toxicol. 2013 Nov-Dec;37(9):642-51. doi: 10.1093/jat/bkt083. Epub 2013 Sep 30. PMID: 24084874.
- Hearn MTW. Ion-pair chromatography on normal and reversed-phase systems. Advance Chromatography. 1980; 18: 59–100.
- Siddiqui MR, AlOthman ZA, Rahman N. Analytical techniques in pharmaceutical analysis: A review. Arabian Journal of Chemistry. 2013.
- Willard H, Merritt L, Dean J, Settle F. Instrumental Methods of Analysis, 7th edn, Wadsworth Publishers, Stamford, CT. 1998