More Information
Submitted: July 18, 2022 | Approved: August 09, 2022 | Published: August 11, 2022
How to cite this article: Khan AU, Ilyas M, Zamel D, Khan S, Ahmad A, et al. Bio-inspired fabrication of zinc oxide nanoparticles: Insight into biomedical applications. Ann Adv Chem. 2022; 6: 023-037.
DOI: 10.29328/journal.aac.1001028
Copyright License: © 2022 Khan AU, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Bio-inspired fabrication of zinc oxide nanoparticles: Insight into biomedical applications
Atta Ullah Khan1*, Muhammd Ilyas1, Doaa Zamel2, Suliman Khan3, Abbas Ahmad3, Fazeela Kaneez4, Sakina Abbas5, Syeda Armana Zaidi6, Hikmat Ullah7, Faisal Adnan8, Shehryar Khan3, Fazal Rahman9 and Subhan Ullah Khan1
1Department of Biotechnology, University of Malakand Chakdara, Pakistan
2Department of Environmental Engineering, Institute of Urban Environment, CAS, China
3Department of Biotechnology, Abdul Wali Khan University, Pakistan
4Institute of Physics, University of Sindh, Pakistan
5Department of Life sciences, Karakoram International University, Gilgit, Pakistan
6Department of Pharmacy, Federal Urdu University of Arts Science & Technology, Karachi,Pakistan
7Department of Microbiology, University of Science and Technology, Kohat, Pakistan
8Department of Botany, Abdul Wali Khan University, Pakistan
9Department of Biotechnology, Quaid E Azam University, Islamabad, Pakistan
*Address for Correspondence: Atta Ullah Khan, Department of Biotechnology, University of Malakand Chakdara, Pakistan, Email: [email protected]
Nanotechnology is starting the characterization, fabrication, and possible applications of numerous materials at the Nano-scale. Over the last few eras, nanomaterials provide a platform for researchers from diverse arenas due to the high surface-to-volume ratio and other novels, and new significant belongings. Zinc oxide nanoparticles are receiving diverse biomedical applications because of their distinctive antimicrobial, antioxidant, anticancer, antifungal, antileishmanial, anti-larvicidal, wound healing, anticholinergic, and anti-diabetic properties. Different physical and chemical approaches have been used to synthesize zinc oxide nanoparticles, but these methods cause ecotoxicity and are time-consuming and costly. Therefore, there is a need for more eco-friendly, cost-effective, and safe methods. Such biogenic Zinc oxide nanoparticles offer more advantages over other physiochemically synthesized methods. In this review, we have summarized the recent literature for the understanding of the green synthesis of Zinc oxide nanoparticles, their characterization, and their various biomedical applications.
In the United States, the National Nanotechnology Initiative started at the commencement of 2000, had systematized the exploration and to develop nanotechnology. Nanomaterials are commonly stated that minor entities having single or many exterior sizes in size range from 1-100 nm. By these sizes, constituents reveal a unique performance in contrast to greater atoms of a similar conformation [1]. Due to the smaller size, they reveal phenomenal unique features and uses in the field of biotechnology, medicine, drug delivery, sensors, and DNA labeling [2,3]. Which are treated as a bridge between bulk material, atomic, and molecular structures [4]. Different routes are used to synthesize metal nanoparticles [5]. They can be synthesized biologically and chemically. Most frequently the chemical methods are used to synthesize metal nanoparticles [6,7] but the ratio of production of toxic chemicals and byproducts is high or requires high pressure and temperatures. This is also the problem with the physical process. Biosynthesis of nanoparticles using plant extracts provides a facile and ‘green’ method of synthesis [8,9].
The characteristics of these nanostructures were the growth mechanisms, fabrication methods, and potential application. The factors affecting the growth mechanisms, the crystallographic natures, growth models of anisotropic nanostructures, and growth of nanocrystals. The differential thermal expansion, the existence of lattice mismatch, and high deposition temperature have affected the uniform deposition of nanoparticles on substrates and caused heteroepitaxy, which can lead to forming a defective nanostructure [10]. The presence of neutral stance levels of apparent state acceptor and donor field makes the characterization of surface state, constructed on photoluminescence and cathode luminescence likely, henceforward, the ease of starting electrons collecting and hole collecting nano interactions for nanostructures short of p-n junctions [11]. The growth of nanostructures throughout fabrications is determined by altered situations which may affect the structural growth along a given direction and result in, unlike growth characteristics and morphologies. The growth way may depend on the surface energy, which tends to direct growth more, along with the facets with higher energy to the detriment of surfaces with lower energy [10].
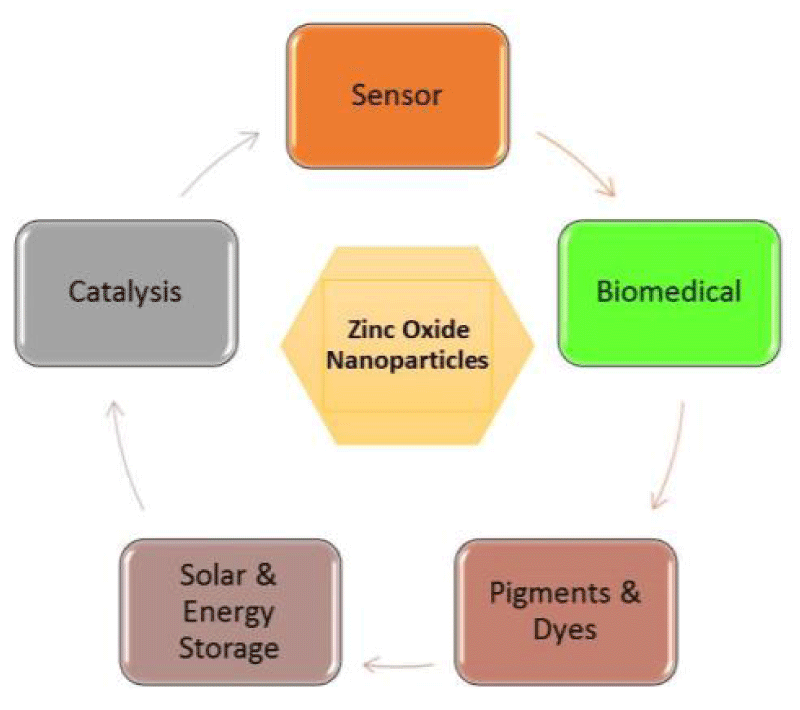
Among various nanoparticles, zinc oxide nanoparticles (ZnO-NPs) have received more importance [12,13]. Zinc oxide performs an active role in various regulatory functions and is involved in the identification of and response in plants to abiotic stresses. It has been identified that zinc has a vital role in the protection of plant cells against oxidative stresses and the management of reactive oxygen species [14]. Applications of these nanostructures are seen in sensors and antibacterial properties (Figure 1) [8]. ZnO-NPs are inorganic nanoparticles that offer larger material properties with functional flexibility [15]. The nature of ZnO-NPs, they are white crystalline powders which insoluble in water. Additionally, the significant feature of ZnO is due to its wide bandgap energy and it belongs to the II-IV group of n-type semiconductors. ZnO has a high excitation binding energy of 60 meV and a wide semiconductor bandgap of 3.37 eV [10].
Figure 1: General applications of ZnO-NPs.
In recent decades ZnO-NPs as an imperative ceramic material that is used in entirely diverse industrial areas such as cosmetic materials medication, concrete, different microorganism, textile, and automotive industries. The ZnO-NPs are widely wont to treat a range of different skin conditions and have anti-cancer properties. However, ZnO-NPs have arisen as an appropriate utensil in drug delivery and sensing horizons [16]. The review aims to summarize the green and biological synthesis methods, characterization and growth mechanism of ZnO-NPs, and investigation of their biomedical applications.
Nanoparticles synthesis
Different approaches can be employed for the synthesis of nanoparticles. These approaches are divided into two classes’ i.e. top-down approach and the bottom-up approach. A top-down approach is a destructive approach in which larger molecules are decomposed into smaller ones, and these smaller molecules are then converted into suitable nanoparticles. While the bottom up is a building approach that involves the assembly of atomic size to form nano-sized particles [17]. These two approaches are further divided into different classes. The Top-down synthesis process includes Chemical etching, Mechanical milling, Laser ablation, Sputtering, and Electro-explosion. While the Top- bottom approach is divided further, such as Biological, and non-biological synthesis. The biological synthesis of NPs from Plant, Microorganisms, and other biological sources while the non-biological method includes the Template support synthesis, Flame spraying synthesis, Chemical vapor deposition, and Spinning Atomic condensation. While in this review, we will only focus on the green synthesis of ZnO-NPs nanoparticles and their biological applications.
Green synthesis of ZnO-NPs (Biological methods)
Nanoparticles are fabricated by physical, chemical, and biological methods. Conventionally, ZnO-NPs are fabricated by physical and chemical methods, which produce a higher production rate and better size control of nanoparticles. However, these fabrication methods are considered to be discouraging due to high capital cost, energy-intensive, timing consumption, and use of toxic and hazardous chemicals [18,19]. Moreover, earlier studies show that the chemical synthesis of nanoparticles is toxic and less biocompatible [20]. Hence physical and chemical approaches have limited nanoparticles’ clinical and biomedical applications. To synthesize NPs, there is a need to explore and grow safer, environmentally friendly, economical, and biocompatible alternatives. The green process of NPs has emerged in recent years through the use of biologically mediated approaches as an alternative to traditional physical and chemical processes [21]. As safe alternatives to chemical methods, biological approaches using microorganisms and plants or plant extracts for the synthesis of metal nanoparticles have been proposed. Several biological systems, including plants, bacteria, fungi, and yeast, have been safely used in biogenic nanoparticle synthesis [22].
Plant-mediated synthesis of zinc oxide nanoparticles: Plants have rich genetic diversity in terms of biomolecules and metabolites like carbohydrates proteins, vitamins, phenols, coenzymes, and flavonoids. These plant metabolites have functional groups like carbonyl, hydroxyl, and amine. Metal ions react with functional groups and reduce their size into the Nano range [23,24]. More specifically, flavonoids contain many functional groups, and the -OH group of flavonoids is thought to be primarily responsible for the reduction of NPs metal ions [25]. These molecules not only assist in the creation of nano-scale ions but also play a key role in the capping of nanoparticles which is required for biocompatibility and stability. Phenolic compounds, sterols, and alkaloids act as a reducing agent in a single reaction and reduce metal ions to NPs [26].
Plants/plant extracts have been widely used for the fabrication of biogenic nanoparticles and are considered the most suitable source due to their easy availability, safety, and cost-effectiveness as compared to bacteria, algae, and other biomolecules. A broad range of biomolecules such as proteins, carbohydrates, enzymes, alkaloids, flavonoids, phenolic acids, and various other molecules in plants plays an important role in the reduction, capping, and stabilization of synthesis of nanoparticles. These molecules react with various zinc salts which leads to the production of ZnO NPS. To date, various plant species have been used for the synthesis of ZnO NPs [27]. The general protocol for the green synthesis of ZnO NPs has various steps. The NPs synthesis mechanism comprises three principal phases: (a) activation phase: the phase of activation comprising the reduction of metal ions and subsequent nucleation of the reduced atom; (b) production phase: the production phase involving spontaneous assemblies and further reducing metal particles by small, adjacent nanoparticles; a process called the Ostwald rifting, which increases thermodynamic stability; and (c) termination phase: in termination phase the final size of Nanoparticles are determined. Primarily, the bio-reducing agents present in the plant extract bind to the metal ions forming metal ions þ metabolites complexes. The resulting complexes allow the metal ions to be decreased to metal atoms and then the reduced metal nanoparticles to be nucleated. The individual small nanoparticles merge into large particles to take on a stable size and shape through a coarsening process. When the growth phase extends, nanoparticles continue adding and attain different sizes and shapes including nanorods, nanotubes, hexahedrons, nano-prisms, and various other nanotubes [28]. In the final phase, which is heavily influenced by the stabilization capability of plant extract, the final confirmation has been acquired. If the nanoparticles’ surface energy is very high, which makes them less stable, they will change their shape to obtain a more stable morphology [29]. Table 1 represents various plants/ plant extracts used for the synthesis of ZnO NPs. ZnO-NPs have been produced using Trifolium pratense flower extract which contains various metabolites such as anthocyanin, phenolic acids and small amounts of tannins, carotene, essential oils and vitamin C. ZnO NPs that are synthesized from T. Pratense demonstrates effective antibacterial activity against strains of S. aureus, E. coli and P. aeruginosa [30]. The synthesis of ZnO-NPs was also investigated using Aloe barbadensis leaf extract with variable shapes in the 8-18nm dimension range. The synthesized NPs have been investigated to check antibacterial activity against E.coli and S. aureus Aureus bacteria and it was concluded that cellular and tissue damage was caused by these biosynthesized ZnO-NPs in both bacteria and thus ZnO-NPs with these properties are considered nano-antibiotics and are more effective than conventional antibiotics against the bacterial strain [31].
| Table 1: Plant-mediated synthesis of zinc oxide nanoparticles. | |||||||
| Specie name | Family | Part use | Phytochemical Constituent | Shape | Size nm | Characterization | Ref |
| Trifolium pratense | Fabaceae | Flower | Flavonoids, Phenolic Acids, Tannins | Spherical | 60–70 nm | XRD, SEM, EDX, UV–Vis, FT-IR | [32] |
| Azadirachta indica | Meliaceae | leaves | Flavonoids, Steroids, Carbohydrates, Glycosides, Antiquinone, Terpenoides And Alkaloids, |
Hexagona, Buds, Cones, Bundles, And Closed Pine Cone | 28 nm | PXRD, FTIR, XPS, UV–Vis | [35] |
| Aloe vera | Liliaceae | Leaves | Glycosides, Antiquinones, Phlobatannins, Carbohydrates, Alkaloids, Terpenes, Saponins, Tannins, Steroids, Flavonoids |
Spherical, Oval And Hexagonal |
8–18 nm | XRD, UV vis, SEM, FTIR HRTEM, | [36] |
| Polygala tenuifolia | Polygalaceae | Roots | Saponins, Oligosaccharide Esters, Xanthones, Oleic Acid | Spherical | 33.03–73.48 nm | UV–Vis, FT-IR, TEM, SEM,AAS | [37] |
| Ficus religiosa | Moraceae | Leaf extract | Alkaloids, Flavonoids, And Terpenoids | Spherical | 76.21 nm | FE-SEM, DLS, XRD, UV–vis, FTIR, EDX, | [38] |
| Pongamia pinnata | Legumes | Fresh Leaf extract | Spherical, Hexagonal, Nano Rod | 32 nm | UV–vis, SEM XRD, TEM, EDX | [39] | |
| Carissa EDULIS | Apocynaceae | Fruits | Acids, Saponin Glycosides, Alkaloids, Tannins, And Terpenoids | Flower Shaped | 50-55 nm | UV-Vis, SEM FT-IR, HR-TEM, and XRD | [40] |
| Camellia sinensis | Theaceae | waste | Steroids, Terpenoids, Carotenoids, Flavonoids, Alkaloids, Tannins, And Glycosides | Spherical | 88 nm | XRD, UV-Vis, FT-IR, AFM | [41] |
| Citrus aurantifolia | Rutaceae | Fruits and peel | Flavonoids, Flavones, Limonoids, Triterpenoid, And Naringenin | Spherical And Pyramids | 50 – 200 nm | XRD, FE-SEM, | [42] |
| Anisochilus carnosus | Lamiaceae | leaves | Alkaloids, Steroids, Glycosides, Flavonoids, And Tannins | Spherical | 20-40 nm | UV–vis, TEM, FTIR, XDR | [43] |
| Rosa canina | Rosaceae | Fruit extract | Polyphenols, Vitamin C, Flavonoids | Spherical | 25-204 nm | DLS, XRD, SEM | [44] |
| Cocus Nucifera | Arecaceae | fresh flower extract | Terpenoids, Resins, Alkaloids, Steroids And Glyco-Sides |
Spherical And Predominantly Hexagonal Without Any Agglomeration | 30–50 nm | FT-IR, SEM, UV-vis | [45] |
| Calatropis Gigantea | Apocynaceae | Fresh leaves | Alkaloids, Flavonoids, Tannins, Glycosides, Terpenoids, And Saponins, | Spherical Shaped Forming Agglomerates | 30–35 nm | SEM, XRD, | [46] |
| Plectranthus amboinicus | Lamiaceae | Leaf extract | Alkaloids, Glycosides, Carbohydrates, Proteins, Flavonoids, Amino Acids, Quinone, Tannins, Terpenoids, And Phenolic Compounds |
Rod Shape Nanoparticle With Agglomerate | 50–180 nm | SEM, TEM, XRD, FTIR, UV-vis | [47] |
| Gossypium | Malvaceae | Cellulosic fiber | Alkaloid, Flavonoid, Saponins, And Cyanogenic Glycosides | Wurtzite, Nano Rod, Spherical, | 13 nm | XRD, SEM, EDX, FT-IR | [48] |
| Solanum lycopersicum | Solaneseace | Leaf extract | Amino Acid, Terpenoids Steroids, Saponins, Flavonoids, Phenols, Carbohydrates, And Essential Oil, |
Spherical | 20–40 nm | UV–vis, DLS FT-IR, HR-TEM, XRD, FE-SEM | [49] |
| Caralluma Fimbriata | Apocynaceae | Aerial part | Glycosides, Polyphenols | Hexagonal | 30 nm | UV-vis, XRD, FTIR | [50] |
| Vitex negundo | Lamiaceae | Leaf extract | Carbohydrates, Anthraquinones, Amino Acids, Saponins, Tannins, Flavonoids And Phenolic Compounds. | Spherical | 75-80 nm | SEM, EDX, XRD | [39] |
| E. crassipes | Pontederiaceae | Leaf extract | Phenols, Alkaloids Protein, Amino Acids, Flavonoids, Carbohydrate, Glycosides, Terpenoids, And Tannins | Spherical | 32 nm | UV-vis, TEM, XRD, SEM, EDX | [51] |
| Nephelium lappaceum L. | Sapindaceae) | Fruit peels | Acids, Flavonoids, Carbohydrates, Protein | Needle-Shaped Forming Agglomerate | 50.95 nm | XRD, SEM, TEM | [52] |
| Coccinia indica | Cucurbitaceae | Leaves, stem, roots, fruits | Phenolic Compounds Carbohydrate, Terpenoids Oil And Fats, Flavonoids, Saponins, Alkaloids, Tannins And Proteins | Hexagonal | 10.4 nm | XRD, FESEM, FTIR, TEM, SEM, | [53] |
| Passiflora caerulea | Passifloraceae | Leaf extract | Flavonoids, Indole Alkaloids, And Maltol, | - | 30–50 nm | EDAX, FTIR, TEM,SEM, UV-vis, AFM | [54] |
| Artocarpus gomezianus | Moraceae | Fruit | Secondary Metabolites Of Tannins Flavonoids And Steroids. | Hexagonal Wurtzite | 30–40 nm | PXRD, SEM,TEM, FTIR, UV–Vis | [55] |
| Rhamnus virgata | Rhamnaceae | Leaf extract | Anthraquinone, Lactone, Phenanthrene, Flavonol, And Xanthone And | Hexagonal, Triangle | ~20 nm | TEM, SEM,FITR,UV-vis | [56] |
| Tecoma castanifolia | Bignoniaceae | Leaf | Alkaloids, Phenols, Flavonoids, And Quinones Saponins | Spherical | 70–75 nm | UV–Vis, EDX TEM, FTIR and XRD | [57] |
| Tamarindus indica | Fabaceae | Pulp extract | Alkaloid, Reducing Sugar, Tannin, Flavonoid, Saponin, Glycoside, Anthraquinone, Phenols And Terpenoid |
Hetero Structures | 19-37 nm | XRD, FTIR, TEM, SEM, | [58] |
| Cassia densistipulata taub | Fabaceae | Flower | Aglycone, Sitosterol, Glycoside Derivative | Spherical | 30 nm | FT-IR, SEM, UV-Vis, | [59] |
| Calliandra haematocephala | Fabaceae | Leaves | Alkaloids, Tannins, Cardiac Glycosides, Saponi Ns, And Flavonoids | Hexagonal Wurtzite Structure | 19.45 nm | XRD, UV-Vis SEM, TEM, | [60] |
| Moringa oleifera | Moringaceae | Leaves | Alkaloids, Glucosinolates, Saponins, Steroids, Phenolic Acids, Flavonoids Tannins, And Terpenes. | Spherical | 6–20 nm | UV-Vis, XRD, FE-SEM, EDX, FT-IR, PL | [61] |
| Aloe vera | Liliaceae | Freeze-dried | Glycosides, Carbohydrates, Alkaloids, Terpenes, Saponins, Tannins, Steroids, Flavonoids |
Spherical, Hexagonal | 25–65 nm | SEM & TEM | [62] |
| Catharanthus roseus | Apocynaceae | Leaves | Alkaloids, Flavonoids | Spherical | 23-27 nm | XRD, EDAX, FT-Raman Spectroscopy, SEM, | [63] |
| Agathosma betulina | Rutaceae | Leaf | Limonene, Psi-Diosphenol Menthone, And Pulegone | Quasi-Spherical | 15 nm | FITR, SEM, TEM, XRS, | [64] |
| Olea Europea | Oleaceae | Leaf | Flavonoids, Biophenols, Secoiridoids, Flavanones,Iridoids, Triterpenes, Isochromans, Benzoic Acid Derivatives, | Zinc Nano Sheets | 18–30 nm | FITR, SEM, XRD | [65] |
| Hibiscus rosa-Sinensis | Malvaceae | Leaf | Tannins, Phenols, Anthraquinones, Quinines, Alkaloids, Flavonoids, Saponins, Terpenoids, Protein, Cardiac Glycosides, Carbohydrates, Mucilage, Reducing Sugars, Essential Oils And Steroid | Spherical Aggregates | > 70 nm | XRD, SEM | [66] |
| Calotropis procera | Apocynaceae | Milky latex | Flavonoids, Cardenolides, And Saponins |
Spherical, Granular | 5-40 nm | XRD,PL, SEM, TEM, UV-Vis | [67] |
| Phaseolus vulgaris | Fabaceae | seed | Alkaloids, Carbohydrates, Anthocyanins, Catechins, Fiber, And Flavonoids Phytic Acid, Saponins, Quercetin, Steroids, Tannins, And Terpenoids | Spherical | 20 nm | EDXRF, SEM, XRD, | [68] |
| Corriandrum sativum | Apiaceae | Leaves | Alkaloids, Terpenoids, Flavanoids, Saponin, Carbohydrates, Tannins, And Sterols | Wurtzite Hexagonal | 66 nm | XRD, FTIR SEM, EDAX | [69] |
| Coffee | Rubiaceae | Powder | Phenolics, Terpenoids Flavonoids, Saponins, And Steroids | Hexagonal | 4.6 nm | DRUV-Vis, XRD, TGA | [70] |
| Nephelium lappaceum | Sapindaceae | Peel | Flavonoids, Terpenes, Tannins, And Steroids | Needle Like | 50 nm | XRD, FTIR, SEM, AFM | [52] |
| Anisochilus carnosus | Lamiaceae | Leaves | Steroids, Alkaloids, Flavonoids, Glycosides, Tannins, Carbohydrates, Oils, And Fats | Spherical | 20-40 nm | UV–vis-DRS, SEM, TEM, FT-IR, XRD, FE-SEM | [43] |
| Aspalathus Linearis | Fabaceae | Flowers | Aspalathin | Spherical | 1-8 nm | EDS, XPS, PL | [71] |
| S. album | Santalaceae | Leaves | Terpenoids, Phenolics Saponin, And Tannins | Nano Rods | 70–140 nm | DLS, SEM, TEM, | [72] |
| Saraca asoca | Fabaceae | Flower extract | Flavonoids, Lignin, Terpenoid, Cardiac Glycosides, Phenolic Compounds, And Tannins | Roughly Spherical | 7–19 nm | XRD, TEM, SEM, UV vis, EDS | [73] |
| Sedum alfredii | Crassulaceae | Leaf | Flavonoid, Phenol, And Flavonol | Hexagonal Wurtzite, Pseudo-Spherical | 53.7 nm | XRD, TEM, SEM, EDS | [74] |
| Boswellia ovalifoliolata | Burseraceae | Stem bark | Boswellic Acid, Terpenoids I.E. Tetracyclic Triterpenes And Pentacyclic Triterpenes |
Spherical | 20 nm | TEM, UV-vis, LSPR, FTIR, XRD | [75] |
| Physalis alkekengi L. | Solanaceae | Seeds | Flavonoids, Withanolides, Phenylpropanoids, Physalins And Alkaloids, | Triangular And Elongated | 50–200 nm | XRD, SEM, EDS, EDX, TEM | [76] |
| Mimosa pudica | Fabaceae | Leaves | Alkaloids, Tannin And Proteins | Wurtzite | 2.71 nm | XRD, DRUV-Vis | [70] |
| Jacaranda mimosifolia | Bignoniaceae | Fruit | Tannins, Oleanolic, Flavonoids, Coumarins Terpenes, Ursolic Acid And Steroids | Spherical | 2-4 nm | XRD, TEM, HRTEM, GC–MS, UV-vis, FITR | [77] |
Euphorbia prolifera leaf extract has also been used to form ZnO-NPs as a reduction and stabilization agent. The deterioration of harmful and toxic organic dyes in wastewater is a major concern and has always been the subject of chemists’ attention over the past few years. Because of their chemical and biological stability, it is very hard to extract dyes by natural processes. NPs synthesized with Euphorbia prolifera leaf extract demonstrated strong catalytic activity for Methylene blue and Congo red degradation in the presence of NaBH4 at room temperature in water [32]. Spherical ZnO-NPs synthesized from 25-40 nm rambutane (Nephelium lappaceum L) peel extract showed photocatalytic activity against methyl orange dye and this dye degradation showed that bio-synthesized ZnO-NPs can effectively degrade 83.99 percent under UV light within 120 min [33]. Sharma et al. synthesized ZnO-NPs using the biological method of using Carica papaya milk latex and ZnO variable-shaped nano-flowers. Due to some favorable properties, such as the ability to minimize recombination of the electron-hole pair, surface defect, crystallite size, texture, and large energy gap, it was found. Excellent photocatalytic activity and exceptional antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus were found in these ZnO-NPs [34]. Table 1 contained various examples of plant-mediated synthesis of ZnO-NPs.
Fungal mediated synthesis of zinc oxide nanoparticles: ZnO-NPs are fabricated by using fungi as the living system. Fungi can be utilized under controlled environments and have huge potential for intracellular and extracellular synthesis of metallic NPs. Due to large-scale production, convenient downstream processing, and cost-effectiveness, extracellular synthesis of NP from fungi is of major use due to its better tolerance and the metal bioaccumulation properties, fungal strains are selected over bacteria. Various fungal species such as Fusarium semi- tectum, Fusarium oxysporum, Pleurotus sojarcaju, Aspergillus fumigates, Penicillium brevicompactu, Clostridium Versicolor, and Alternaria alternate are being used for the synthesis of nanoparticles; thus an attempt to synthesize ZnO-NPs with Aspergillus niger is being made. Using UV-vis absorption, EDX, and SEM analyses, confirmed the ZnO-NPs particles synthesis. These nanoparticles also report antimicrobial and cytotoxic effects [78].
In the extracellular synthesis of ZnO-NPs, the synthesized nanoparticles were stabilized by fungus-released protein. The process is simple, cheap, and safe to use, produces large amounts of stable nanoparticles, and has no contaminants or pollution. This method was useful for zinc oxide extracellular synthesis but is slow and requires specific conditions for successful nanoparticle synthesis. After a period of time, the synthesized NPs decompose, and the solution to this problem is a challenge for scientists [79].
The analytical techniques like XRD, SEM, and EDX characterized the synthesized nanoparticles. In the XRD study, zinc oxide NPs are 40 nm in size. The SEM study shows that the ZnO-NPs have a hexagonal shape of 66 nm average size, which deviates from the agglomerated XRD results. The polarity and electrostatic interactions between zinc oxide NPs are the reason for this agglomeration. The EDX confirmed that the synthesized nanoparticles are pure, and the impurities in the sample are only traced. ZnO is used in agriculture, pharma, drugs, ceramics, glass, cement, oil, paint and lubricant, glue, plastic, battery, and fire retardants. In the fields of nanotechnology, UV detectors, nanoscale detectors, and actuators, zinc oxide is showing promising signs. In chip production, it would replace silicon. Due to its dual semiconductor and piezoelectric properties, the ZnO has a bright future. There are many aspects of biological approaches which still need to be found and manipulated in the field of biogenic synthesis of microbial metallic NP.
Another study reported Aspergillus fumigatus mycelia synthesized ZnO-NPs. DLS analyzes have revealed an average size range of NPs of 1.2 to 6.8. The average NP height confirmed by AFM was 8.56 nm. For 90 days it was a particle size of >100 nm but after 90 days they were formed into a medium size 100 nm agglomerate, which suggested 90 days of stability to formed NPs [21]. SEM was confirmed in the size between 54.8 – 82.6 nm for the NPs synthesized in the Aspergillus terreus family of Trichocomaceae and the average size of 29 nm was calculated with the Debye-Sherrer equation using XRD results. FTIR studies confirmed the presence of primary alcohol, primary or secondary amine, amide, and aromatic nitro compounds in NPs [80]. A similar range of 15 to 25 nm, as confirmed by SEM, TEM, and XRD analysis, was presented in NPs synthesized by Candida albicans [81]. Aspergillus species were widely used for the synthesis of ZnO NPs and in most cases, spherical NPs synthesized by fungal strains. Table 2 provides a brief account of commonly used ZnO-NPs synthesis funguses.
| Table 2: Fungus-mediated synthesis of zinc oxide nanoparticles. | |||||
| Fungal stain | Family | Characterization | Size nm | Shape | Ref |
| Aspergillus fumigatus TFR-8 | Trichocomaceae | DLS, SEM | 1.2–6.8 nm (DLS), 100 (agglomerate) | Oblate spherical and hexagonal forms aggregate | [83] |
| Aspergillus aeneus | Trichocomaceae | SEM, | 100~140 nm | Spherical | [82] |
| Fusarium spp. | Nectriaceae | TEM, XRD, FTIS, EDS | > 100m, | Triangle | [84] |
| Candida albicans | Saccharomycetaceae | XRD, SEM, TEM | 25nm | Quasi-spherical | [81] |
| Aspergillus fumigatus JCF | Trichocomaceae | UV–Vi, SEM, FTIS | 60~80 nm | Spherical | [21] |
Kalpana, et al. 2018 evaluate the extracellular summary of ZnO-NPs using Aspergillus niger culture filtrate. There was a maximum absorption rate of 320 nm for the obtained UV visible spectrum of the culture filters. In the FTIR strip, the carboxylic acid appearance and the strong aromatic ring could lead to a ZnO NPs synthesis. Studies of the antimicrobial activity of ZnO-NPs coated fabric showed a reduction of viable E. coli and S. aureus have it. The treatment with 100 μl ZnO-NPs resulted in 90 percent discolors of Bismarck brown dye. In comparison to dye with ZnO-NPs, the germination (percent) of the seeds of plants was lower with the raw dye. This study shows that ZnO-NPs have been found to be mediated in A. niger through a synthesis in degrading coloration which can be incorporated into cotton textiles since the antibacterial activity is revealed [82]. Table 2 contained the example of fungus-mediated synthesized ZnO-NPs.
Bacterial mediated synthesis of zinc oxide nanoparticles: Synthesis of NP using the bacteria is a green approach, but has many drawbacks such as microbial screening is a time-consuming procedure, diligent control for the cultivation of bacteria, and a complete procedure to protect against contamination. The biological synthesis of micro-organism NP seems to be more environmentally friendly and has gained a lot of interest in comparison with plants, provided that without seasonal or geographical constraints micronutrients can be easily cultivated. In addition, the use of bacteria for ZnO-NPs biosynthesis has important advantages because of the fabrication of supernatant functional biomolecules, which can reduce metal ions to metal NPs [85,86]. In addition, bacteria’s cell biomass can act as a nano-factory in ZnO-NPs due to the existence of a functional bacterial cell group, which reduces the metal ions to metal NPs [87,88].
Prokaryotes and eukaryotes, including bacteria, fungi, and yeast, are used to synthesize ZnO-NPs through the use of intracellular or extra-cellular microbial cells or enzymes, proteins, and other biomolecules compounds. However, the properties of nanoparticles (NPs) rely on their size and type which make them unique to different applications. ZnO-NPs have antimicrobial properties. However, by controlling their reaction conditions the necessary size and shape of NPs can be obtained by optimizing the synthesis mediated by microbes. The synthesis of ZnO-NPs with different chemical and physical techniques should be noted. These approaches, however, are costly and unfriendly to the environment. The microbes that mediate ZnO-NP synthesis have therefore evolved rapidly, as alternatives to chemical and physical methods have become safer, environmentally friendly, non-toxic, and biocompatible. In addition, zinc is more efficient in the form of NPs than its bulk equivalents and has been studied in a variety of possible applications including in the animal industry. In particular, ZnO-NPs have grown as potential antimicrobial agents with the advent of multidrug-resistant strains. This is primarily because of their superior properties in the fight against a wide variety of pathogens [21].
Shabib, et al. 2016 investigated that Probiotic lactic acid bacteria (LAB) are of great interest among bacteria used for the synthesis of ZnO-NPs because of their non-pathogenic and beneficial properties. LAB is a gram-positive bacterium that consists of several biostructures and functional groups with a thick cell wall. The metal ion ligands act as functional groups and promote ZnO-NP formation. LAB also secretes multiple enzymes acting as a ZnO-NPs reducer and stabilizer. Several studies have therefore been performed to evaluate the effectiveness of probiotic LAB in mediating the biosynthesis of ZnO-NPs by cell-biomass or cell-free supernatant. Nevertheless, it remains uncharged to date the potential to use both routes to mediate ZnO-NP biosynthesis [89].
ZnO nanoflowers were synthesized by B. licheniformis through an eco-friendly approach which showed photocatalytic activity and degraded Methylene blue dye. These nanoflowers showed enhanced photocatalytic activity as compared to already present photocatalytic substances and it has been presumed that larger oxygen vacancy in the synthesized nanoparticles imparts the property of enhanced photocatalytic activity. Photocatalysis generates active species by absorption of light which degrades the organic waste material and thus can be used as an effective bioremediation tool. Nanoflowers synthesized using B. licheniformis were 40 nm in width and 400 nm in height [83]. Rhodococcus is able to survive in adverse conditions and it has the ability to metabolize hydrophobic compounds thus, can help in biodegradation [90]. Spherical-shaped NPs had been synthesized using Rhodococcus pyridinivorans and Zinc Sulphate as a substrate which showed a size range of 100–130 nm confirmed through FE-SEM and XRD Analysis. It also demonstrated the presence of Phosphorus compound, secondary sulphonamide, monosubstituted alkyne, β-lactone, amine salt, amide II stretching band, enol of 1-3-di ketone, hydroxy aryl ketone, amide I bending band, alkane, and mononuclear benzene band confirmed through FTIR analysis [91]. ZnO was used as a substrate to synthesize ZnO-NPs through A. hydrophilla. NPs synthesized showed a size range of 42–64 nm, confirmed through AFM and XRD analysis with varied shapes like oval and spherical [92]. Singh, et al. compared the antioxidant activity of bare ZnO-NPs and Pseudomonas aeruginosa rhamnolipid stabilized NPs and it had been found that rhamnolipid stabilizes the ZnO-NP because it is tough to form micelle aggregates on the surface of carboxymethyl cellulose [93] and it acts as a better capping agent because of its long carbon chain. It showed the formation of spherical-shaped NP with a nano-size of 27–81 nm confirmed through TEM, XRD, and DLS analysis [94]. Tables 3, 4, and 5 contained the Bacterial; actinomycetes, and Algae mediated synthesized ZnO-NPs.
| Table 3: Bacterial mediated synthesis of zinc oxide nanoparticles. | ||||||
| Bacterial stain | Family | Gram +/Gram - | Size nm | Shape | Characterization | Ref |
| Bacillus licheniformis | Bacillaceae | Gram + | 200 with nano pedals 40 in width and 400 in length | Nano-flowers | TEM,XRD,TGA | [91] |
| Lactobacillus casei | Lactobacillaceae | Gram + | 20–50 | spherical | FT-IR, EDX, XRD, FESEM, TEM | [95] |
| Staphylococcus aureus | Staphylococcaceae | Gram + | 10-50 | Acicular | XRD, FTIR, TGA, UV–vis, EDX, FESEM | [96] |
| Pseudomonas aeruginosa | Pseudomonadaceae | Gram - | 35–80 (TEM), 27 (XRD), 81 (DLS) | Spherical | TEM, XRD, DLS | [94] |
| Lactobacillus Sporogenous |
Bacillaceae | Gram + | 5–15 (TEM), 11 (XRD) | Hexagonal unit cell | TEM, XRD SEM, UV-vis | [97] |
| Rhodococcus pyridinivorans |
Nocardiaceae | Gram + | 100–120 (FE-SEM), 120–130 (XRD) | Hexagonal phase, roughly spherical | FE-SEM, XRD | [93] |
| Serratia ureilytica | Enterobacteriacea | Gram - | 170–250 (30 min), 300–600 (60 min), 185–365 (90 min) [SEM] |
Spherical to nanoflower shaped | SEM,TEM | [98] |
| Halomonas elongate | Halomonadaceae | Gram - | 10-61 | Multiform | FTIR, SEM,TEM, XRD, UV–Vis | [99] |
| Lactobacillus johnsonii | Lactobacillaceae | Gram + | 4~9 | Spherical | SEM, TEM, UV-Vis | [100] |
| Bacillus megaterium | Bacillaceae | Gram + | 45~95 | Rod and cubic | UV-Vis, FTIR, XRD, FESEM | [101] |
| Sphingobacterium thalpophilum | Sphingobacteriaceae | Gram - | 40 | Triangle | FTIR, TEM, UV-Vis | [102] |
| Aeromonas hydrophila | Aeromonadaceae | Gram - | 57.7 | Spherical | TEM,XRD, SEM | [103] |
| Table 4: Synthesis of Zinc oxide nanoparticles from actinomycetes. | ||||
| actinomycetes | Characterization | Size | Shape | Ref |
| Streptomyces sps | UV-VIS, Scanning Electron Microscope (SEM), FTIR spectroscopy |
15-24 | Spherical | [104] |
| Nocardiopsis sp. GRG1 (KT23540) | UV–vis absorption spectra, Fourier transform infrared (FTIR), X-ray diffraction (XRD) pattern, Raman spectra, energy-dispersive X-ray spectroscopy (EDS) and morphologically using scanning electron microscopy (SEM). | 10-14 | Hexagonal phase, roughly spherical | [105] |
| Streptomyces sp. HBUM171191 | UV-Vis Spectrophotometer | 10-20 | Shperical | [106] |
| Sphingobacterium thalpophilum | X-ray diffractometer (model: X’Pert Pro Pan alytical), Field emission scanning electron microscope (FESEM; model: Supra 55) with EDAX (model: Ultra 55), y Ultraviolet visible spectrophotometer (UV-Vis; model: Lamda 35). | 40 | Triangle | [107] |
| Staphylococcus aureus | UV-visible spectroscopy, transmission and scanning electron microscopy, X-ray diffraction analysis | 10-50 | Acicular | [108] |
| Rhodococcus pyridinivorans NT2 | (FE-SEM), (XRD) | 100-120 | Hexagonal phase, roughly spherical | [109] |
| Table 5: Algal-Mediated synthesis using Zinc Oxide Nano Particle. | ||||
| Algae Strains | Size (nm) | Functional Group | Shape | Ref |
| Chlamydomonas reinhardtii (Chlamydomonaceae) |
21 (XRD),55–80 (HR-SEM) | C = O stretching, N-H bending band of amide I and amide II, C = O stretch of zinc acetate, C-O-C of polysaccharide | Nanorod, porous nanosheet | [110] |
| Sargassum muticum (Sargassaceae) |
42 (XRD), 30–57 (FE-SEM) | Asymmetric stretching band of the sulfate group, an asymmetric C-O band associated with C-O-SO3 & -OH group, sulfated polysaccharides | Hexagonal | [111] |
| S. myriocystum (Sargassaceae) |
20–36 (AFM), 46.6 (DLS) | O-H and C = O stretching band, carboxylic acid | Spherical, triangle, hexagonal | [112] |
| Anabaena cylindrica (Nostocaceae) |
40-60nm (FESEM and TEM) | stretching vibration of bonded and non-bonded O-H groups, asymmetric – CH2, symmetric –CH3, and CH2- stretching vibrations, C-O-C stretching, stretching C-N bond | Road Like | [113] |
Biomedical applications
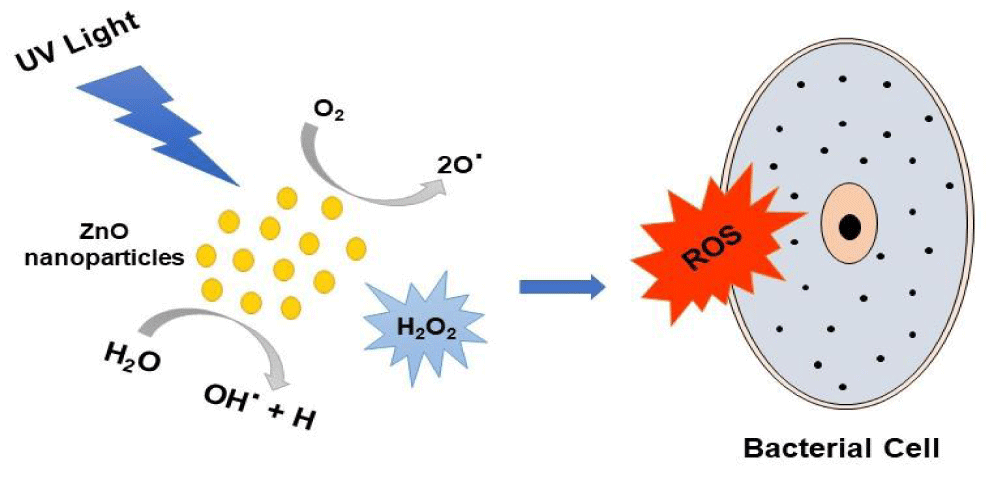
Antibacterial activity: The misuse and unregulated use of antibiotics have developed bacterial resistance which became a challenge for the treatment of several infectious diseases. In this regard, nanotechnology offers a new therapeutic strategy to treat diseases that developed from antibiotic-resistant pathogenic bacteria [114]. Zinc oxide (ZnO) is a bio-safe material that has photo-oxidizing and photocatalysis properties that makes them effective on biological species. Furthermore, Zinc oxide has been investigated as an associate degree agent in medication when used in each microscale and nanoscale formulations [115]. Researchers studied the photoactivation of ZnO-NPs while exposed to UV light at 254 nm for 30 min in a UV transilluminator, and the growth analysis was performed with 2 and 5 mM concentrations using similar conditions. The results showed that the antibacterial activity of ZnO-NPs in the dark is less than that in ambient laboratory conditions [116]. In most of the studies, it was found that the mechanism of Zinc oxide nanoparticles could be due to the formation of reactive oxygen species (ROS), Figure 2. Researchers study the antibacterial effect of zinc oxide nanoparticles against Staphylococcus aureus (S. aureus) bacteria and they proved that small-sized zinc oxide nanoparticles showed a better antibacterial effect. The mechanism was explained by the generation of H2O2 in S. aureus cells which leads to cell death [117]. Similarly, Zinc oxide nanoparticles exhibited antibacterial activity against E.coli 0157:H7 and the data showed complete inhibition from a concentration of 12 mmol/ L. This inhibition occurred due to cellular disruption which lead to cell death [118]. A further study explained that the antibacterial properties of Zinc oxide nanoparticles against S. aureus were developed due to the formation of intracellular ROS which was found to be the reason behind the bacterial cell death [119]. Another study revealed that zinc oxide nanoparticles have antibacterial activity against Campylobacter jejuni. From the results, scanning electron microscopy confirmed that, after exposure to 0.5 mg/ mL of zinc oxide nanoparticles for 16 h, morphological changes were developed which caused a transformation from spiral shapes into coccoid forms in C. jejuni [120]. Recently, Hameed, et al. [121] studied the effect of zinc oxide nanoparticles immobilized on fabricated neodymium which exhibited unique antibacterial activity against extended-spectrum β-lactamases (ESBLs)-generating E. coli and Klebsiella pneumoniae bacteria. In this study, it was noticed that the cell membrane developed more distortion, besides bacterial cell shrinkage when compared to free zinc oxide nanoparticles.
Figure 2: Mechanism of Antibacterial Activity of ZnO-NPs.
Anticancer activity: Cancer results from unrestricted proliferation and immortality of cells due to impairment in the uncontrolled program of cell death (apoptosis) [122]. The elimination of cancer cells inside the human body ever requires searching for effective scopes to bring up new therapeutic strategies. As known, present chemotherapy agents are not effectively treating cancer and fail to bring a complete anticancer effect because these chemotherapeutic substances cannot differentiate between cancerous and normal cells. Thereby, they may lead to normal cell toxicity and dangerous side effects such as; suppression of bone marrow function, damage to hair and nails, neurotoxicity, and cardiomyopathy [123]. Therefore, searching for alternative therapeutic agents that have a higher degree of selectivity and affinity toward cancer cells is highly required. This belief opened the avenue for exploring the potential of zinc oxide nanoparticles as effective anticancer material. The following reports present the possible therapeutic approaches brought by zinc oxide nanoparticles. The genotoxicity of zinc oxide nanoparticles was evaluated in a previous study while DNA damage occur causing reduction in cell viability upon exposure to ZnO-NPs. Furthermore, ZnO-NPs were found to induce oxidative stress which was indicated by a decrease in glutathione (59% and 51%); catalase (64% and 55%), and superoxide dismutase (72% and 75%) at a concentration of 0.8 and 0.08 g/ml respectively [124]. Further in vivo study in mice investigated the oral toxicity of ZnO-NPs while the results came up with a cellular injury after oral demonstration of ZnO-NPs (300 mg/kg) for 14 days due to the accumulation of nanoparticles in the liver. This liver injury was assessed by the increased serum levels of both alanine aminotransferase (ALT) and alkaline phosphatase (ALP) liver enzymes besides pathological lesions in the liver. Furthermore, ZnO-NPs were found to increase oxidative stress inside liver cells and subsequently cause DNA damage and apoptosis [125]. Earlier in 2016, Pandurangan, et al. [126] studied the anticancer effect of zinc oxide nanoparticles (10 nm) in human cervical carcinoma cells (Hela) with different concentrations ranging from 0.001 to 0.06 mg/mL for 48 hrs. Results showed a reduction in cell viability in a range from 5% to 50% in HeLa cells while they did not cause a great effect on normal Madin-Darby Canine Kidney (MDCK) cells as their cell viability results at 0.06 mg/mL was 95%. Another study in 2017, Manshian, et al. [127] assessed the anticancer activities of free zinc oxide nanoparticles and Fe-doped zinc oxide nanoparticles (10 - 20 nm), and the results revealed that doping zinc oxide nanoparticles with iron (2 - 10%) diminished the ionization of Zn into Zn2+ ions and reduced ZnO cytotoxicity towards normal cells (murine mesenchymal stem cells and human bronchial epithelial cells) and cancer cells (HeLa and murine lung squamous carcinoma cells). Later in 2018, Heng and Zhang [128] studied the anticancer effects of both Chitosan coated and non-coated zinc oxide nanoparticles in HeLa cells while the concentration of ZnO ranged from 0.1 to 75 µg/mL for 24 hrs. From the results, both coated and uncoated ZnO-NPs exhibited low cytotoxicity as they reduced cell viability by a percentage less than 10% when using a concentration of 1 µg/mL. Thereby, chitosan-coated zinc oxide nanoparticles which are positively charged caused elevated cytotoxicity via easier cellular internalization. Subsequently, they increased reactive oxygen species formation and apoptosis whereas complete cell death occurred at a concentration of 75 µg/mL. Recently in 2019 [128], a concentration of ZnO-NPs ranging from (20 - 200 ug/mL) was used for the treatment in SiHa cells for 24 hrs and showed cytotoxicity with an IC50 value of 35 μg/mL. Complete cell death was obtained at 200 ug/mL which was the maximum concentration. However, ZnO-NPs did not show exhibit any toxicity towards normal peripheral blood mononuclear cells at a concentration up to 200 ug/mL. Another study on HeLa cells evaluated the anticancer activity of green synthesized ZnO-NPs in normal human embryonic kidney (HEK-293) cells. Herein, cell toxicity was observed when treated with ZnO-NPs with a concentration ranging from 6.25-200 μg/mL for 24 hrs due to the production of ROS [129]. Another study showed the anticancer effect of ZnO-NPs and 55 % of cell death happened while applying the maximum concentration of 20 μM/mL. They revealed no cytotoxicity toward normal MCF-10A cells [130]. In 2020, Zinc oxide nanoparticles’ anticancer mechanism against A549 cells was studied while ZnO-NPs showed cytotoxicity in A549 cells at a concentration ranging from 1 to 25 ug/mL for 24 hrs. Thereby, the mechanism was by induction of apoptosis and depressing the migration rate in MCF-7 cells [131].
Antifungal activity: A prior study was conducted to test the viability of the pathogenic yeast, Candida albicans (C. Albicans) after exposure to ZnO-NPs. Here, inhibition of over 95% in the growth of C. Albicans was obtained at the minimal fungicidal concentration of ZnO which is 0.1 mg/ ml. Furthermore, visible light excited ZnO-NPs and enhanced yeast cell death. It was suggested that cell death was due to the involvement of reactive oxygen species; including hydroxyl radicals and singlet oxygen [132]. Further study in 2017, greenly synthesized zinc oxide nanoparticles from Ziziphus nummularia leaf extract were characterized by various spectral analyses, and their antifungal (anticandidal) activity was evaluated against multidrug-resistant isolates. The results were better than four standard azole antibiotics and ZnO-NPs further exhibited a cytotoxic effect against HeLa cells [133]. In 2018 Sardella et al., developed a rapid and accurate technique to assess the efficacy of novel antifungal agents such as zinc oxide nanoparticles against three isolates. A. alternate, B. cinerea, and R. stolonifer. Results did not achieve significant growth inhibition in A. alternate and B. cinerea at 15 mM of ZnO-NPs concentration while in the case of R. stolonifer the maximum inhibition occurred at that concentration [134]. Another study was performed on metal oxide nanomaterials (zinc oxide, magnesium oxide, and ZnO: MgO and ZnO: Mg(OH)2 composites) and explored their antifungal activities against the fungal strain of C. gloeosporioides obtained from papaya and avocado fruits. The results showed that all nanoparticles significantly inhibited the germination of conidia and caused structural damage to the fungus cells at the tested concentrations [135]. Later in 2019, Miri, et al. studied the antifungal activity of ZnO which has been synthesized greenly by Prosopis fractal aqueous extract, and the characterization have been done by UV–Vis absorption, Fourier-Transform Infrared spectroscopy (FT-IR), Raman, Powder X-ray Diffraction (PXRD), Transmission Electron Microscopy (TEM), Field Emission Scanning Electron Microscopy (FESEM) and Energy Dispersive X-ray analysis (EDX). Herein, results have shown the antifungal activity of ZnO-NPs against Candida albicans and the minimum fungicidal concentration (MFC) was 256 μg/ml [136]. Extra studies focused on three-particle morphologies of Zinc oxide and in vitro study of their antifungal activity against three phytopathogenic fungi species; Fusarium oxysporum lycopersici, Fusarium solani, and Colletotrichum gloeosporioids. Herein, ZnO particle shapes were nanoparticles, lamellar platelets, and hexagonal rods. Furthermore, they were obtained by colloidal and hydrothermal synthesis techniques, the synthesis parameters, and methodology. Electron microscopy imaging techniques were used for the determination of ZnO-NPs size and morphology. Results came up with that ZnO with platelets shaped particles have better antifungal inhibition activity than rods and nanoparticles and it inhibited the growth of Fusarium solani by a maximum percentage of 65% [137]. Reactive DC magnetron was used for the production of thin films of zinc oxide and tested against the pathogenic fungus Candida albicans. The results revealed the successful formation of stoichiometric ZnO thin films and their significant antifungal activity with inhibition of cell growth by 68%. From this paper, it was suggested that the formation of mesoporous films of ZnO enhanced its antifungal activity against Candida albicans when compared with ZnO-NPs [138]. A recent study in 2020 focused on the antifungal effect of ZnO-NPs and Nystatin on gene expression of SAP1-3 in Candida albicans (C. Albicans) and the results showed a significant reduction in gene expression for both ZnO-NPs and Nystatin [139]. Another study was performed against Penicillium chrysogenum to exploit the antifungal activity of ZnO-NPs. In that study, biogenesis and capping processes of the synthesized nanoparticles were performed using the functional fungal extracellular enzymes and proteins. The ability of the bio-secreted proteins cape ZnO have been demonstrated to change their particle shape to hexagonal and spherical ZnO-NPs with particle size at 9.0 - 35.0 nm which exhibited good antimicrobial activities against some phytopathogenic fungal strains and some bacterial species. Highlighted from that study, the green synthesized ZnO-NPs have been considered as smart nano-material for the application in the medical field and treatment of some pathogenic microbes [140].
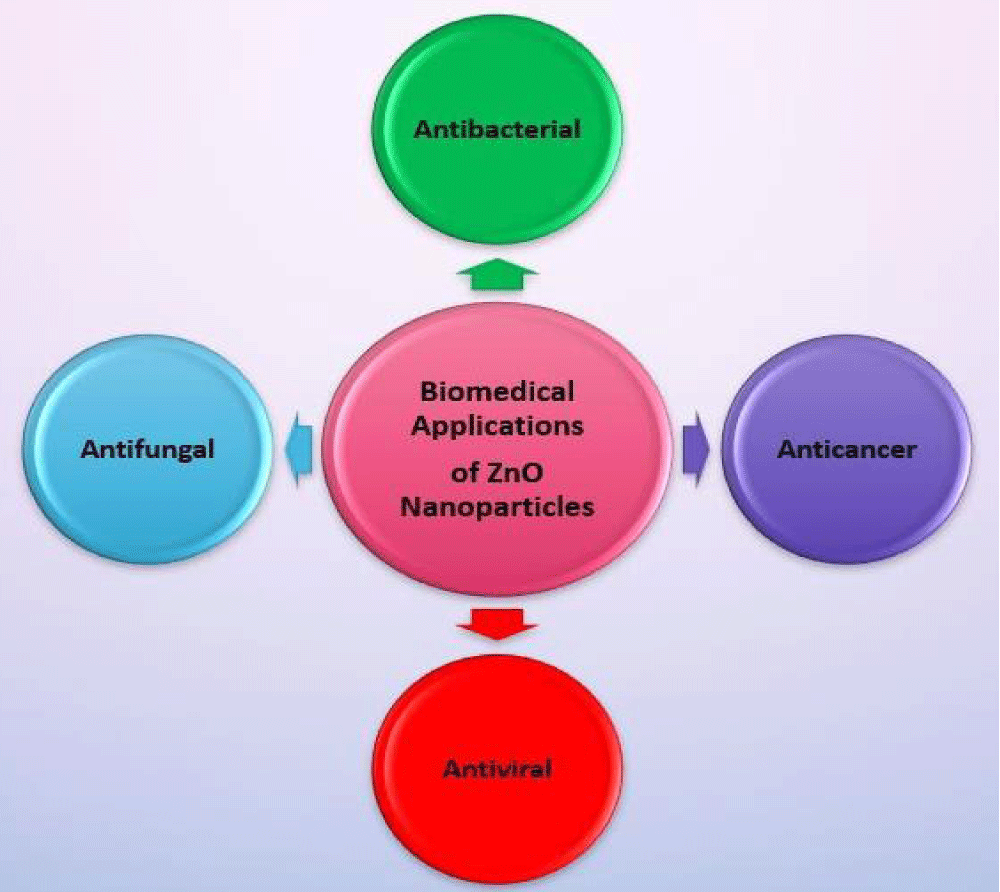
Antiviral activity: Zinc oxide nanoparticles have been used for developing effective treatment of several viruses and here previous studies will be demonstrated. Kumar et al., investigated the use of ZnO-NPs which were synthesized by precipitation method and characterized by Transmission Electron Microscope (TEM), Fourier transform infrared (FTIR), and zetadynamic light scattering for treatment of Chikungunya (CHIKV). After treatment of cells with zinc oxide nanoparticles, results revealed that ZnO-NPs could interact with viruses, and this caused inhibition of viral growth and cell death. Therefore, ZnO could be considered a new effective antiviral drug against the chikungunya virus though further tests on animals are still required for approval [141]. In another study, Hydroxyl group rich ZNPs (H-ZNPs), oleic acid modified ZNPs (OA-ZNPs) and chitosan-Zinc nanoparticles (C-ZNPs) were chemically synthesized and their characterization was performed by ultra-violet (UV) and Fourier transforms infrared (FTIR) spectroscopy, transmission electron microscope (TEM) and selected area electron diffraction (SAED). Moreover, their antiviral activity was assessed and the results suggested that surface-modified ZnO-NPs could obstruct the virus infectivity potential by the virus neutralization rather than interfering with cellular targets. In this regard, the electrostatic interference of H-ZNPs and physical entrapment exhibited by C-ZNPs is more significant than the hydrophobic interaction with OA-ZNPs [142]. The further study focused on evaluating the antiviral activity of zinc oxide nanoparticles and PEGylated zinc oxide nanoparticles against the H1N1 influenza virus. Herein, results showed that the PEGylated and unPEGylated ZnO-NPs both have antiviral activity against the H1N1 virus at the highest non-toxic concentrations with inhibition percentages of 94.6% and 52.2%, respectively [143]. In a 2020 recent study, green synthesized zinc oxide nanoparticles from aqueous leaf extract of Mentha spicata were tested against Tobacco mosaic virus (TMV) and the results confirmed the antiviral effect of ZnO-NPs with reduction of viral accumulation reached 90.21% [144]. The biomedical applications are shown in Figure 3.
Figure 3: Various Biomedical applications of ZnO-NPs.
Other biological applications: Zinc oxide nanoparticles have other biological applications including bioimaging where ZnO-NPs have many desirable optical properties which make them suitable for application for optical imaging [145]. Furthermore, ZnO-NPs are unique nanomaterials for application in drug delivery due to their high surface area, versatile surface chemistry, phototoxic effect, and others [146]. Moreover, ZnO-NPs also exhibit semiconducting properties, high catalytic efficiency, and strong adsorption capability which enables them to be reliable for biosensors applications. Besides they have high isoelectric point ~9.5 which are versatile for some proteins adsorption for instance; certain enzymes and antibodies by electrostatic interaction [147].
The green synthesized ZnO-NPs have diverse biological and biomedical applications. Traditionally, nanoparticles are synthesized by either physical or chemical methods, which not only leads to environmental toxicity but also costly and energy-intensive labor. ZnO-NPs are synthesized by a green route using the extracts of different plants/parts of plants, microorganisms, and other biological molecules such as gelatin, oleic acid, and starch. The bio-mediated ZnO-NPs are environmentally friendly, facile in terms of synthesis, cost-effective, most fascinating, and biocompatible. The FDA has ZnO-NPs on its list of safe substances. However, additional research is still needed on a few important ZnO-NPs-related concerns, such as the following: The limitations of ZnO-NPs toxicity toward biological systems remain a contentious issue in recent research, and there is a lack of evidence-based randomized studies specifically examining therapeutic roles in improving anticancer, antibacterial, anti-inflammatory, and antidiabetic activities. Additionally, there is a lack of understanding of corresponding animal studies about its anticancer, antibacterial, anti-inflammatory, and antidiabetic activities. The usage of ZnO nanoparticles in biomedical diagnostic and therapeutic sectors may be better clarified and understood through the conduct of further studies centered on the aforementioned problems. We think that nanomaterials would significantly advance the ZnO nanoparticles and are anticipated to make more intriguing contributions in various domains of medicine.
Conflict of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
- Zuverza-Mena N, Armendariz R, Peralta-Videa JR, Gardea-Torresdey JL. Effects of Silver Nanoparticles on Radish Sprouts: Root Growth Reduction and Modifications in the Nutritional Value. Front Plant Sci. 2016 Feb 16;7:90. doi: 10.3389/fpls.2016.00090. PMID: 26909084; PMCID: PMC4754487..
- Gnanasangeetha D, Sarala Thambavani D. One pot synthesis of zinc oxide nanoparticles via chemical and green method. Res J Mater Sci, 2013. 2320: p. 6055.
- Ullah Khan A, Chen L, Ge G. Recent development for biomedical applications of magnetic nanoparticles. Inorg Chem Commun. 2021 Dec;134:108995. doi: 10.1016/j.inoche.2021.108995. Epub 2021 Oct 8. PMID: 34658663; PMCID: PMC8500685..
- Gnanasangeetha D, Sarala Thambavani D One pot synthesis of zinc oxide nanoparticles via chemical and green method. Research Journal of Material Sciences, 2013. 2320: p. 6055.
- Satyavani K, Ramanathan T, Gurudeeban S. Green synthesis of silver nanoparticles by using stem derived callus extract of bitter apple (Citrullus colocynthis). Digest Journal of Nanomaterials and Biostructures, 2011. 6(3): p. 1019-1024.
- Noruzi M, Zare D, Khoshnevisan K, Davoodi D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc. 2011 Sep;79(5):1461-5. doi: 10.1016/j.saa.2011.05.001. Epub 2011 May 6. PMID: 21616704..
- Ilyas, M, et al. Biological synthesis of titanium dioxide nanoparticles from plants and microorganisms and their potential biomedical applications. Inorganic Chemistry Communications, 2021. 133: p. 108968.
- Varghese E, George M. Green synthesis of zinc oxide nanoparticles. International Journal of Advance Research In Science And Engineering, 2015. 4(01): p. 307-314.
- Bessa MJ, Brandão F, Viana M, Gomes JF, Monfort E, Cassee FR, Fraga S, Teixeira JP. Nanoparticle exposure and hazard in the ceramic industry: an overview of potential sources, toxicity and health effects. Environ Res. 2020 May;184:109297. doi: 10.1016/j.envres.2020.109297. Epub 2020 Feb 24. PMID: 32155489..
- Ossai CI, Raghavan N. Nanostructure and nanomaterial characterization, growth mechanisms, and applications. Nanotechnology Reviews, 2018. 7(2): p. 209-231.
- Hasegawa H, et al. Dynamics and control of recombination process at semiconductor surfaces, interfaces and nano-structures. Solar Energy, 2006. 80(6): p. 629-644.
- Srivastava V, Gusain D, Sharma YC. Synthesis, characterization and application of zinc oxide nanoparticles (n-ZnO). Ceramics International, 2013. 39(8): p. 9803-9808.
- Khan AU, Khan T, Khan MA, Nadhman A, Aasim M, Khan NZ, Ali W, Nazir N, Zahoor M. Iron-doped zinc oxide nanoparticles-triggered elicitation of important phenolic compounds in cell cultures of Fagonia indica. Plant Cell Tissue Organ Cult. 2021;147(2):287-296. doi: 10.1007/s11240-021-02123-1. Epub 2021 Jun 16. PMID: 34149126; PMCID: PMC8206870.
- Alharby HF, et al. Impact of application of zinc oxide nanoparticles on callus induction, plant regeneration, element content and antioxidant enzyme activity in tomato (Solanum lycopersicum Mill.) under salt stress. Archives of Biological Sciences, 2016. 68(4): p. 723-735.
- Tyagi PK, Shruti SV, Ahuja A. Synthesis of metal nanoparticals: A biological prospective for analysis. International Journal of Pharmaceutical Innovations, 2012. 4: p. 48-60.
- Mirzaei H, Darroudi M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceramics International, 2017. 43(1): p. 907-914.
- Khan I, Saeed K, Khan I. Nanoparticles: Properties, applications and toxicities. Arabian journal of chemistry, 2019. 12(7): p. 908-931.
- Shamim A, Abid MB, Mahmood T. Biogenic synthesis of zinc oxide (ZnO) nanoparticles using a fungus (Aspargillus niger) and Their Characterization. International Journal of Chemistry, 2019. 11(2): p. 119-126.
- Agarwal H, Kumar SV, Rajeshkumar S, A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resource-Efficient Technologies, 2017. 3(4): p. 406-413.
- Hosseini MR, Sarvi MN. Recent achievements in the microbial synthesis of semiconductor metal sulfide nanoparticles. Materials Science in Semiconductor Processing, 2015. 40: p. 293-301.
- Mohd Yusof H, Mohamad R, Zaidan UH, Abdul Rahman NA. Microbial synthesis of zinc oxide nanoparticles and their potential application as an antimicrobial agent and a feed supplement in animal industry: a review. J Anim Sci Biotechnol. 2019 Jul 9;10:57. doi: 10.1186/s40104-019-0368-z. PMID: 31321032; PMCID: PMC6615095..
- Alagumuthu G, Kirubha R. Green synthesis of silver nanoparticles using Cissus quadrangularis plant extract and their antibacterial activity. International Journal of Nanomaterials and Biostructures, 2012. 2(3): p. 30-33.
- Salman SA, Kuroda K, Okido M. Preparation and Characterization of Hydroxyapatite Coating on AZ31 Mg Alloy for Implant Applications. Bioinorg Chem Appl. 2013;2013:175756. doi: 10.1155/2013/175756. Epub 2013 Feb 21. PMID: 23533371; PMCID: PMC3600141..
- >Kavitha K, et al. Plants as green source towards synthesis of nanoparticles. Int Res J Biol Sci, 2013. 2(6): p. 66-76.
- Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, Kalinina NO. "Green" nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae. 2014 Jan;6(1):35-44. PMID: 24772325; PMCID: PMC3999464..
- Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A. Antimicrobial Activities of Leaf Extracts of Guava (Psidium guajava L.) on Two Gram-Negative and Gram-Positive Bacteria. Int J Microbiol. 2013;2013:746165. doi: 10.1155/2013/746165. Epub 2013 Oct 20. PMID: 24223039; PMCID: PMC3817707..
- Parashar V, et al. Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Digest Journal of Nanomaterials & Biostructures (DJNB), 2009. 4(1).
- Kim JH, Cho H, Ryu SE, Choi MU. Effects of metal ions on the activity of protein tyrosine phosphatase VHR: highly potent and reversible oxidative inactivation by Cu2+ ion. Arch Biochem Biophys. 2000 Oct 1;382(1):72-80. doi: 10.1006/abbi.2000.1996. PMID: 11051099..
- Ali K, Dwivedi S, Azam A, Saquib Q, Al-Said MS, Alkhedhairy AA, Musarrat J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J Colloid Interface Sci. 2016 Jun 15;472:145-56. doi: 10.1016/j.jcis.2016.03.021. Epub 2016 Mar 12. PMID: 27031596..
- Dobrucka R, Długaszewska J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J Biol Sci. 2016 Jul;23(4):517-23. doi: 10.1016/j.sjbs.2015.05.016. Epub 2015 May 31. PMID: 27298586; PMCID: PMC4890195..
- Sharma S, et al. The effect of shape and size of ZnO nanoparticles on their antimicrobial and photocatalytic activities: a green approach. Bulletin of Materials Science, 2020. 43(1): p. 1-10.
- Momeni SS, Nasrollahzadeh M, Rustaiyan A. Green synthesis of the Cu/ZnO nanoparticles mediated by Euphorbia prolifera leaf extract and investigation of their catalytic activity. J Colloid Interface Sci. 2016 Jun 15;472:173-9. doi: 10.1016/j.jcis.2016.03.042. Epub 2016 Mar 19. PMID: 27038280.
- Karnan T, Selvakumar SAS. Biosynthesis of ZnO nanoparticles using rambutan (Nephelium lappaceumL.) peel extract and their photocatalytic activity on methyl orange dye. Journal of molecular Structure, 2016. 1125: p. 358-365.
- Sharma S. ZnO nano-flowers from Carica papaya milk: degradation of Alizarin Red-S dye and antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus. Optik, 2016. 127(16): p. 6498-6512.
- Raphael E. Phytochemical constituents of some leaves extract of Aloe vera and Azadirachta indica plant species. Global Advanced Research Journal of Environmental Science and Toxicology, 2012. 1(2): p. 014-017.
- Nagajyothi PC, Cha SJ, Yang IJ, Sreekanth TV, Kim KJ, Shin HM. Antioxidant and anti-inflammatory activities of zinc oxide nanoparticles synthesized using Polygala tenuifolia root extract. J Photochem Photobiol B. 2015 May;146:10-7. doi: 10.1016/j.jphotobiol.2015.02.008. Epub 2015 Feb 25. PMID: 25777265.
- Wu D, He J, Jiang Y, Yang B. Quality analysis of Polygala tenuifolia root by ultrahigh performance liquid chromatography-tandem mass spectrometry and gas chromatography-mass spectrometry. J Food Drug Anal. 2015 Mar;23(1):144-151. doi: 10.1016/j.jfda.2014.07.009. Epub 2014 Dec 5. PMID: 28911438; PMCID: PMC9351754.
- Heer A, Mansoori S. Biosynthesis and characterization of zinc oxide nanoparticle using ficus religiosa leaves extract. World Journal of Pharmaceutical Research, 2017. 6(10): p. 818-826.
- Sundrarajan M, Ambika S, Bharathi K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Advanced powder technology, 2015. 26(5): p. 1294-1299.
- Fowsiya J, Madhumitha G, Al-Dhabi NA, Arasu MV. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J Photochem Photobiol B. 2016 Sep;162:395-401. doi: 10.1016/j.jphotobiol.2016.07.011. Epub 2016 Jul 14. PMID: 27434698.
- Al-Ogaidi I. Camellia sinensis (Green Tea) mediated synthesis of zinc oxide nanoparticles and detect its antibacterial activity against Escherichia coli, Staphylococcus aureus and Acinetobacter baumannii. J Biotechnol Res Center, 2017. 11: p. 34-40.
- Çolak H, Karaköse E. Green synthesis and characterization of nanostructured ZnO thin films using Citrus aurantifolia (lemon) peel extract by spin-coating method. Journal of Alloys and Compounds, 2017. 690: p. 658-662.
- Anbuvannan M. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Materials Science in Semiconductor Processing, 2015. 39: p. 621-628.
- Jafarirad S, Mehrabi M, Divband B, Kosari-Nasab M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater Sci Eng C Mater Biol Appl. 2016 Feb;59:296-302. doi: 10.1016/j.msec.2015.09.089. Epub 2015 Sep 28. PMID: 26652376..
- Priyatharesini PI, Ganesamoorthy R, Sudha R. Synthesis of Zinc Oxide Nanoparticle using Cocos nucifera male Flower Extract and Analysis their Antimicrobial Activity. Research Journal of Pharmacy and Technology, 2020. 13(5): p. 2151-2154.
- Vidya C. Green synthesis of ZnO nanoparticles by Calotropis gigantea. Int J Curr Eng Technol, 2013. 1(1): p. 118-120.
- Fu L, Fu Z. Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceramics International, 2015. 41(2): p. 2492-2496.
- Aladpoosh R, Montazer M. The role of cellulosic chains of cotton in biosynthesis of ZnO nanorods producing multifunctional properties: Mechanism, characterizations and features. Carbohydr Polym. 2015 Aug 1;126:122-9. doi: 10.1016/j.carbpol.2015.03.036. Epub 2015 Mar 27. PMID: 25933530.
- Singh A, Singh NB, Hussain I, Singh H, Yadav V, Singh SC. Green synthesis of nano zinc oxide and evaluation of its impact on germination and metabolic activity of Solanum lycopersicum. J Biotechnol. 2016 Sep 10;233:84-94. doi: 10.1016/j.jbiotec.2016.07.010. Epub 2016 Jul 13. PMID: 27422354.
- Mishra P. Caralluma fimbriata extract induced green synthesis, structural, optical and photocatalytic properties of ZnO nanostructure modified with Gd. Journal of Alloys and Compounds, 2016. 685: p. 656-669.
- Vanathi P. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: a green chemistry approach. Materials Letters, 2014. 134: p. 13-15.
- 52">Yuvakkumar R, Suresh J, Nathanael AJ, Sundrarajan M, Hong SI. Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater Sci Eng C Mater Biol Appl. 2014 Aug 1;41:17-27. doi: 10.1016/j.msec.2014.04.025. Epub 2014 Apr 18. PMID: 24907732.
- Safawo T. Synthesis and characterization of zinc oxide nanoparticles using tuber extract of anchote (Coccinia abyssinica (Lam.) Cong.) for antimicrobial and antioxidant activity assessment. OpenNano, 2018. 3: p. 56-63.
- Santhoshkumar J, Kumar SV, Rajeshkumar S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resource-Efficient Technologies, 2017. 3(4): p. 459-465.
- Anitha R. Cytotoxicity, antibacterial and antifungal activities of ZnO nanoparticles prepared by the Artocarpus gomezianus fruit mediated facile green combustion method. Journal of Science: Advanced Materials and Devices, 2018. 3(4): p. 440-451.
- Iqbal J. Plant-extract mediated green approach for the synthesis of ZnONPs: Characterization and evaluation of cytotoxic, antimicrobial and antioxidant potentials. Journal of Molecular Structure, 2019. 1189: p. 315-327.
- Sharmila G, Thirumarimurugan M, Muthukumaran C. Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchemical Journal, 2019. 145: p. 578-587.
- Thatikayala D. Enhanced photocatalytic and antibacterial activity of ZnO/Ag nanostructure synthesized by Tamarindus indica pulp extract. Journal of Materials Science: Materials in Electronics, 2020. 31(7): p. 5324-5335.
- Kooluru NR, Sharada S. Green synthesis of zinc oxide nanoparticles using flower extract Cassia densistipulata Taub. International Journal of Engineering Research and Development, 2014. 10: p. 16-19.
- Vinayagam R, Selvaraj R, Arivalagan P, Varadavenkatesan T. Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala-mediated zinc oxide nanoflowers. J Photochem Photobiol B. 2020 Jan;203:111760. doi: 10.1016/j.jphotobiol.2019.111760. Epub 2019 Dec 18. PMID: 31884350..
- Elumalai, K., et al., RETRACTED: green synthesis of zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. 2015, Elsevier.
- Qian Y, Yao J, Russel M, Chen K, Wang X. Characterization of green synthesized nano-formulation (ZnO-A. vera) and their antibacterial activity against pathogens. Environ Toxicol Pharmacol. 2015 Mar;39(2):736-46. doi: 10.1016/j.etap.2015.01.015. Epub 2015 Feb 2. PMID: 25723342.
- Bhumi G, Savithramma N. Biological synthesis of zinc oxide nanoparticles from Catharanthus roseus (L.) G. Don. Leaf extract and validation for antibacterial activity. Int J Drug Dev Res, 2014. 6(1): p. 208-214.
- Thema F. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Materials Letters, 2015. 161: p. 124-127.
- Awwad AM, Albiss B, Ahmad AL. Green synthesis, characterization and optical properties of zinc oxide nanosheets using Olea europea leaf extract. Adv. Mater. Lett, 2014. 5(9): p. 520-524.
- Devi RS, Gayathri R. Green synthesis of zinc oxide nanoparticles by using Hibiscus rosa-sinensis. International Journal of Current Engineering and Technology, 2014. 4(4): p. 2444-2446.
- Singh RP. Biological approach of zinc oxide nanoparticles formation and its characterization. Adv. Mater. Lett, 2011. 2(4): p. 313-317.
- Savassa SM, et al. Effects of ZnO nanoparticles on Phaseolus vulgaris germination and seedling development determined by X-ray spectroscopy. ACS Applied Nano Materials, 2018. 1(11): p. 6414-6426.
- Patel K, Vakilwala M. Phytochemical study and bioactivity of solvent extracts on Coriandrum sativum. Int. J. Adv. Res. Biol. Sci, 2016. 3(5): p. 193-199.
- Fatimah I, Pradita RY, Nurfalinda A. Plant extract mediated of ZnO nanoparticles by using ethanol extract of Mimosa pudica leaves and coffee powder. Procedia engineering, 2016. 148: p. 43-48.
- Diallo A. Green synthesis of ZnO nanoparticles by Aspalathus linearis: structural & optical properties. Journal of Alloys and Compounds, 2015. 646: p. 425-430.
- Zhu X, Pathakoti K, Hwang HM. Green synthesis of titanium dioxide and zinc oxide nanoparticles and their usage for antimicrobial applications and environmental remediation, in Green Synthesis, Characterization and Applications of Nanoparticles. 2019, Elsevier. p. 223-263.
- Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol. 2007 Dec 15;41(24):8484-90. doi: 10.1021/es071445r. PMID: 18200883..
- Qu J, Luo C, Hou J. Synthesis of ZnO nanoparticles from Zn-hyperaccumulator (Sedum alfredii Hance) plants. Micro & Nano Letters, 2011. 6(3): p. 174-176.
- Supraja N. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Applied Nanoscience, 2016. 6(4): p. 581-590.
- Qu J, Yuan X, Wang X, Shao P. Zinc accumulation and synthesis of ZnO nanoparticles using Physalis alkekengi L. Environ Pollut. 2011 Jul;159(7):1783-8. doi: 10.1016/j.envpol.2011.04.016. Epub 2011 May 6. PMID: 21549461.
- Sharma, D., et al., Biosynthesis of ZnO nanoparticles using Jacaranda mimosifolia flowers extract: synergistic antibacterial activity and molecular simulated facet specific adsorption studies. Journal of Photochemistry and Photobiology B: Biology, 2016. 162: p. 199-207 .
- Pavani, K., N.S. Kumar, and B. Sangameswaran, Synthesis of lead nanoparticles by Aspergillus species. Polish Journal of Microbiology, 2012. 61(1): p. 61-63.
- Mashrai A, Khanam H, Aljawfi RN. Biological synthesis of ZnO nanoparticles using C. albicans and studying their catalytic performance in the synthesis of steroidal pyrazolines. Arabian Journal of Chemistry, 2017. 10: p. S1530-S1536.
- Kitching M, Ramani M, Marsili E. Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb Biotechnol. 2015 Nov;8(6):904-17. doi: 10.1111/1751-7915.12151. Epub 2014 Aug 26. PMID: 25154648; PMCID: PMC4621444.
- Moreno-Martin G, Pescuma M, Pérez-Corona T, Mozzi F, Madrid Y. Determination of size and mass-and number-based concentration of biogenic SeNPs synthesized by lactic acid bacteria by using a multimethod approach. Anal Chim Acta. 2017 Nov 1;992:34-41. doi: 10.1016/j.aca.2017.09.033. Epub 2017 Sep 28. PMID: 29054148.
- Kalpana V. Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano, 2018. 3: p. 48-55.
- Raliya R, Tarafdar JC. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agricultural Research, 2013. 2(1): p. 48-57.
- Velmurugan P, Shim J, You Y, Choi S, Kamala-Kannan S, Lee KJ, Kim HJ, Oh BT. Removal of zinc by live, dead, and dried biomass of Fusarium spp. isolated from the abandoned-metal mine in South Korea and its perspective of producing nanocrystals. J Hazard Mater. 2010 Oct 15;182(1-3):317-24. doi: 10.1016/j.jhazmat.2010.06.032. Epub 2010 Jun 15. PMID: 20599320..
- Chegeni M, Pour SK, Dizaji BF. Synthesis and characterization of novel antibacterial Sol-gel derived TiO2/Zn2TiO4/Ag nanocomposite as an active agent in Sunscreens. Ceramics International, 2019. 45(18): p. 24413-24418.
- Król A. Mechanism study of intracellular zinc oxide nanocomposites formation. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018. 553: p. 349-358.
- Selvarajan E, Mohanasrinivasan V. Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Materials Letters, 2013. 112: p. 180-182.
- Gnanajobitha G. Fruit-mediated synthesis of silver nanoparticles using Vitis vinifera and evaluation of their antimicrobial efficacy. Journal of Nanostructure in Chemistry, 2013. 3(1): p. 1-6.
- Al-Shabib NA, Husain FM, Ahmed F, Khan RA, Ahmad I, Alsharaeh E, Khan MS, Hussain A, Rehman MT, Yusuf M, Hassan I, Khan JM, Ashraf GM, Alsalme A, Al-Ajmi MF, Tarasov VV, Aliev G. Biogenic synthesis of Zinc oxide nanostructures from Nigella sativa seed: Prospective role as food packaging material inhibiting broad-spectrum quorum sensing and biofilm. Sci Rep. 2016 Dec 5;6:36761. doi: 10.1038/srep36761. Erratum in: Sci Rep. 2017 Feb 09;7:42266. PMID: 27917856; PMCID: PMC5137238.
- Otari S. Green biosynthesis of silver nanoparticles from an actinobacteria Rhodococcus sp. Materials Letters, 2012. 72: p. 92-94.
- Tripathi RM, Bhadwal AS, Gupta RK, Singh P, Shrivastav A, Shrivastav BR. ZnO nanoflowers: novel biogenic synthesis and enhanced photocatalytic activity. J Photochem Photobiol B. 2014 Dec;141:288-95. doi: 10.1016/j.jphotobiol.2014.10.001. Epub 2014 Oct 29. PMID: 25463680.
- Mehta SK, Kumar S, Chaudhary S, Bhasin KK. Effect of Cationic Surfactant Head Groups on Synthesis, Growth and Agglomeration Behavior of ZnS Nanoparticles. Nanoscale Res Lett. 2009 Jul 1;4(10):1197-1208. doi: 10.1007/s11671-009-9377-8. PMID: 20596462; PMCID: PMC2893803.
- Kundu D, Hazra C, Chatterjee A, Chaudhari A, Mishra S. Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J Photochem Photobiol B. 2014 Nov;140:194-204. doi: 10.1016/j.jphotobiol.2014.08.001. Epub 2014 Aug 12. PMID: 25169770.
- Singh BN, Rawat AK, Khan W, Naqvi AH, Singh BR. Biosynthesis of stable antioxidant ZnO nanoparticles by Pseudomonas aeruginosa rhamnolipids. PLoS One. 2014 Sep 4;9(9):e106937. doi: 10.1371/journal.pone.0106937. PMID: 25187953; PMCID: PMC4154833.
- Kouhkan M. Biosynthesis of copper oxide nanoparticles using Lactobacillus casei subsp. casei and its anticancer and antibacterial activities. Current Nanoscience, 2020. 16(1): p. 101-111.
- Mahamuni PP, Patil PM, Dhanavade MJ, Badiger MV, Shadija PG, Lokhande AC, Bohara RA. Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem Biophys Rep. 2018 Dec 12;17:71-80. doi: 10.1016/j.bbrep.2018.11.007. PMID: 30582010; PMCID: PMC6295600.
- Kumari P. Biosynthesized Zinc Oxide nanoparticles control the growth of Aspergillus flavus and its aflatoxin production. International Journal of Nano Dimension, 2019. 10(4): p. 320-329.
- Dhandapani P, Siddarth AS, Kamalasekaran S, Maruthamuthu S, Rajagopal G. Bio-approach: Ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohydr Polym. 2014 Mar 15;103:448-55. doi: 10.1016/j.carbpol.2013.12.074. Epub 2014 Jan 5. PMID: 24528753.
- Taran M, Rad M, Alavi M. Biosynthesis of TiO2 and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. Bioimpacts. 2018;8(2):81-89. doi: 10.15171/bi.2018.10. Epub 2017 Dec 27. PMID: 29977829; PMCID: PMC6026522.
- Al-Zahrani H., A. El-Waseif, and D. El-Ghwas, Biosynthesis and evaluation of TiO2 and ZnO nanoparticles from in vitro stimulation of Lactobacillus johnsonii. J Innov Pharm Biol Sci, 2018. 5: p. 16-20.
- Saravanan M, Gopinath V, Chaurasia MK, Syed A, Ameen F, Purushothaman N. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb Pathog. 2018 Feb;115:57-63. doi: 10.1016/j.micpath.2017.12.039. Epub 2017 Dec 14. PMID: 29248514.
- Ovais M, Khalil AT, Islam NU, Ahmad I, Ayaz M, Saravanan M, Shinwari ZK, Mukherjee S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl Microbiol Biotechnol. 2018 Aug;102(16):6799-6814. doi: 10.1007/s00253-018-9146-7. Epub 2018 Jun 7. PMID: 29882162.
- Jayaseelan C, Rahuman AA, Kirthi AV, Marimuthu S, Santhoshkumar T, Bagavan A, Gaurav K, Karthik L, Rao KV. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim Acta A Mol Biomol Spectrosc. 2012 May;90:78-84. doi: 10.1016/j.saa.2012.01.006. Epub 2012 Jan 9. PMID: 22321514.
- Rajamanickam U. Synthesis of Metal Oxide Nano Particles by Streptomyces Sp for Development of Antimicrobial Textiles. Global journal of biotechnology and biochemistry, 2010. 5: p. 153.
- Maruthupandy M. Antibiofilm activity of zinc oxide nanosheets (ZnO NSs) from Nocardiopsis sp. GRG1 (KT23540) against MDR strains of gram negative Proteus mirabilis and Escherichia coli. Process Biochemistry, 2018. 67.
- Waghmare SS. Biosynthesis and characterization of manganese and zinc nanoparticles. Univ. J. Environ. Res. Technol., 2011. 1: p. 64-69.
- Rajabairavi N. Biosynthesis of Novel Zinc Oxide Nanoparticles (ZnO NPs) Using Endophytic Bacteria Sphingobacterium thalpophilum. 2017. p. 245-254.
- Rauf MA. Biomimetically synthesized ZnO nanoparticles attain potent antibacterial activity against less susceptible S. aureus skin infection in experimental animals. RSC Advances, 2017. 7(58): p. 36361-36373.
- Kundu D, Hazra C, Chatterjee A, Chaudhari A, Mishra S. Extracellular biosynthesis of zinc oxide nanoparticles using Rhodococcus pyridinivorans NT2: multifunctional textile finishing, biosafety evaluation and in vitro drug delivery in colon carcinoma. J Photochem Photobiol B. 2014 Nov;140:194-204. doi: 10.1016/j.jphotobiol.2014.08.001. Epub 2014 Aug 12. PMID: 25169770.
- Rao MD, Gautam P. Synthesis and characterization of ZnO nanoflowers using Chlamydomonas reinhardtii: A green approach. Environmental Progress & Sustainable Energy, 2016. 35(4): p. 1020-1026.
- Azizi S, Namvar F, Mahdavi M, Ahmad MB, Mohamad R. Biosynthesis of Silver Nanoparticles Using Brown Marine Macroalga, Sargassum Muticum Aqueous Extract. Materials (Basel). 2013 Dec 18;6(12):5942-5950. doi: 10.3390/ma6125942. PMID: 28788431; PMCID: PMC5452754.
- Nagarajan S, Arumugam Kuppusamy K. Extracellular synthesis of zinc oxide nanoparticle using seaweeds of gulf of Mannar, India. J Nanobiotechnology. 2013 Dec 3;11:39. doi: 10.1186/1477-3155-11-39. PMID: 24298944; PMCID: PMC3879036.C.
- Banerjee S. Green Synthesis of Zinc Oxide Nanoparticles via Algal Route and its Action on Cancerous Cells and Pathogenic Microbes. Advanced Nano Research, 2020. 3(1): p. 15-27.
- Riduan SN, Armugam A, Zhang Y. Antibiotic resistance mitigation: the development of alternative general strategies. J Mater Chem B. 2020 Aug 5;8(30):6317-6321. doi: 10.1039/d0tb01241f. PMID: 32597439.
- Sharma R, Garg R, Kumari A. A review on biogenic synthesis, applications and toxicity aspects of zinc oxide nanoparticles. EXCLI J. 2020 Sep 22;19:1325-1340. doi: 10.17179/excli2020-2842. PMID: 33192216; PMCID: PMC7658464.
- Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008 Feb;279(1):71-6. doi: 10.1111/j.1574-6968.2007.01012.x. Epub 2007 Dec 11. PMID: 18081843.
- Seil JT, Webster TJ. Antimicrobial applications of nanotechnology: methods and literature. Int J Nanomedicine. 2012;7:2767-81. doi: 10.2147/IJN.S24805. Epub 2012 Jun 6. PMID: 22745541; PMCID: PMC3383293.
- Liu Y, He L, Mustapha A, Li H, Hu ZQ, Lin M. Antibacterial activities of zinc oxide nanoparticles against Escherichia coli O157:H7. J Appl Microbiol. 2009 Oct;107(4):1193-201. doi: 10.1111/j.1365-2672.2009.04303.x. Epub 2009 Apr 17. PMID: 19486396.
- Sirelkhatim, A., et al., Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-micro letters, 2015. 7(3): p. 219-242.
- Xie Y, He Y, Irwin PL, Jin T, Shi X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl Environ Microbiol. 2011 Apr;77(7):2325-31. doi: 10.1128/AEM.02149-10. Epub 2011 Feb 4. PMID: 21296935; PMCID: PMC3067441.
- Hameed AS, Karthikeyan C, Ahamed AP, Thajuddin N, Alharbi NS, Alharbi SA, Ravi G. In vitro antibacterial activity of ZnO and Nd doped ZnO nanoparticles against ESBL producing Escherichia coli and Klebsiella pneumoniae. Sci Rep. 2016 Apr 13;6:24312. doi: 10.1038/srep24312. PMID: 27071382; PMCID: PMC4829841.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646-74. doi: 10.1016/j.cell.2011.02.013. PMID: 21376230.
- Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257-88. doi: 10.1146/annurev.bioeng.9.060906.152025. PMID: 17439359.
- Sharma V, Shukla RK, Saxena N, Parmar D, Das M, Dhawan A. DNA damaging potential of zinc oxide nanoparticles in human epidermal cells. Toxicol Lett. 2009 Mar 28;185(3):211-8. doi: 10.1016/j.toxlet.2009.01.008. PMID: 19382294.
- Sharma V, Singh P, Pandey AK, Dhawan A. Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles. Mutat Res. 2012 Jun 14;745(1-2):84-91. doi: 10.1016/j.mrgentox.2011.12.009. Epub 2011 Dec 17. PMID: 22198329.
- Pandurangan M, Enkhtaivan G, Kim DH. Anticancer studies of synthesized ZnO nanoparticles against human cervical carcinoma cells. J Photochem Photobiol B. 2016 May;158:206-11. doi: 10.1016/j.jphotobiol.2016.03.002. Epub 2016 Mar 9. PMID: 26985734.
- Manshian BB, Pokhrel S, Himmelreich U, Tämm K, Sikk L, Fernández A, Rallo R, Tamm T, Mädler L, Soenen SJ. In Silico Design of Optimal Dissolution Kinetics of Fe-Doped ZnO Nanoparticles Results in Cancer-Specific Toxicity in a Preclinical Rodent Model. Adv Healthc Mater. 2017 May;6(9). doi: 10.1002/adhm.201601379. Epub 2017 Feb 23. PMID: 28230930.
- Singh TA, Das J, Sil PC. Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv Colloid Interface Sci. 2020 Dec;286:102317. doi: 10.1016/j.cis.2020.102317. Epub 2020 Nov 9. PMID: 33212389.
- Nouri M. Toward Real-World BCI: CCSPNet, A Compact Subject-Independent Motor Imagery Framework. arXiv preprint arXiv:2012.13567, 2020.
- Jiang J, Pi J, Cai J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg Chem Appl. 2018 Jul 5;2018:1062562. doi: 10.1155/2018/1062562. PMID: 30073019; PMCID: PMC6057429.
- Mathew EN. Investigating the biophysical, biochemical, and biological activity of anti-cancer zinc oxide nanoparticle and its physiometacomposite (PMC) nanoparticles. 2020.
- Lipovsky A, Nitzan Y, Gedanken A, Lubart R. Antifungal activity of ZnO nanoparticles--the role of ROS mediated cell injury. Nanotechnology. 2011 Mar 11;22(10):105101. doi: 10.1088/0957-4484/22/10/105101. Epub 2011 Feb 2. PMID: 21289395.
- Padalia H, Chanda S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif Cells Nanomed Biotechnol. 2017 Dec;45(8):1751-1761. doi: 10.1080/21691401.2017.1282868. Epub 2017 Jan 31. PMID: 28140658.
- Sardella D, Gatt R, Valdramidis VP. Turbidimetric Assessment of the Growth of Filamentous Fungi and the Antifungal Activity of Zinc Oxide Nanoparticles. J Food Prot. 2018 Jun;81(6):934-941. doi: 10.4315/0362-028X.JFP-17-448. PMID: 29745759.
- la Rosa-García D. Antifungal activity of ZnO and MgO nanomaterials and their mixtures against Colletotrichum gloeosporioides strains from tropical fruit. Journal of Nanomaterials, 2018. 2018.
- Miri A, Mahdinejad N, Ebrahimy O, Khatami M, Sarani M. Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109981. doi: 10.1016/j.msec.2019.109981. Epub 2019 Jul 16. PMID: 31500056.
- Pariona N. Shape-dependent antifungal activity of ZnO particles against phytopathogenic fungi. Applied Nanoscience, 2020. 10(2): p. 435-443.
- Pereira-Silva P. Antifungal activity of ZnO thin films prepared by glancing angle deposition. Thin Solid Films, 2019. 687: p. 137461.
- Hosseini, S.S., et al., Antifungal activity of ZnO nanoparticles and nystatin and downregulation of SAP1-3 genes expression in fluconazole-resistant Candida albicans isolates from vulvovaginal candidiasis. Infection and drug resistance, 2020. 13: p. 385.
- Mohamed AA, Abu-Elghait M, Ahmed NE, Salem SS. Eco-friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol Trace Elem Res. 2021 Jul;199(7):2788-2799. doi: 10.1007/s12011-020-02369-4. Epub 2020 Sep 8. Erratum in: Biol Trace Elem Res. 2020 Sep 25;: PMID: 32895893.
- Kumar R. Virostatic potential of zinc oxide (ZnO) nanoparticles on capsid protein of cytoplasmic side of chikungunya virus. International Journal of Infectious Diseases, 2018. 73: p. 368.
- Farouk F, Sgebl R. Comparing surface chemical modifications of zinc oxide nanoparticles for modulating their antiviral activity against herpes simplex virus type-1. Int. J. Nanopart. Nanotechnol, 2018. 4: p. 21.
- Ghaffari H, Tavakoli A, Moradi A, Tabarraei A, Bokharaei-Salim F, Zahmatkeshan M, Farahmand M, Javanmard D, Kiani SJ, Esghaei M, Pirhajati-Mahabadi V, Monavari SH, Ataei-Pirkooh A. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J Biomed Sci. 2019 Sep 10;26(1):70. doi: 10.1186/s12929-019-0563-4. PMID: 31500628; PMCID: PMC6734352.
- Abdelkhalek A, Al-Askar AA. Green synthesized ZnO nanoparticles mediated by Mentha spicata extract induce plant systemic resistance against Tobacco mosaic virus. Applied Sciences, 2020. 10(15): p. 5054.
- Grumezescu AM. Inorganic Frameworks as Smart Nanomedicines. 2018: William Andrew.
- Zhang Y, Nayak TR, Hong H, Cai W. Biomedical applications of zinc oxide nanomaterials. Curr Mol Med. 2013 Dec;13(10):1633-45. doi: 10.2174/1566524013666131111130058. PMID: 24206130; PMCID: PMC3838497.
- Zhu P. Biomedical applications of functionalized ZnO nanomaterials: from biosensors to bioimaging. Advanced Materials Interfaces, 2016. 3(1): p. 1500494.