More Information
Submitted: August 12, 2022 | Approved: September 07, 2022 | Published: September 08, 2022
How to cite this article: Vayenas CG. Electrochemical promotion of catalysis. Ann Adv Chem. 2022; 6: 048-050.
DOI: 10.29328/journal.aac.1001031
Copyright License: © 2022 Vayenas CG. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Electrochemical promotion of catalysis
Constantinos G Vayenas1,2*
1Academy of Athens, Panepistimiou 28 Ave, 10679, Athens, Greece
2University of Patras, Caratheodory 1 St, GR-26504 Patras, Greece
*Address for Correspondence: Constantinos G Vayenas, Academy of Athens, Panepistimiou 28 Ave, 10679, Athens, Greece, Email: [email protected]
The Electrochemical Promotion of Catalysis (EPOC) or Non-Faradaic Electrochemical Promotion of Catalysis (NEMCA effect) is a phenomenon observed as a reversible change in catalytic rate (i.e. no net charge transfer rate) of a chemical reaction occurring on a catalyst film (or supported dispersed catalyst) deposited on an ionically conducting or mixed electronically-ionically conducting solid electrolyte support upon the application of an electrical potential between the catalyst and a second conductive film deposited on the solid electrolyte support.
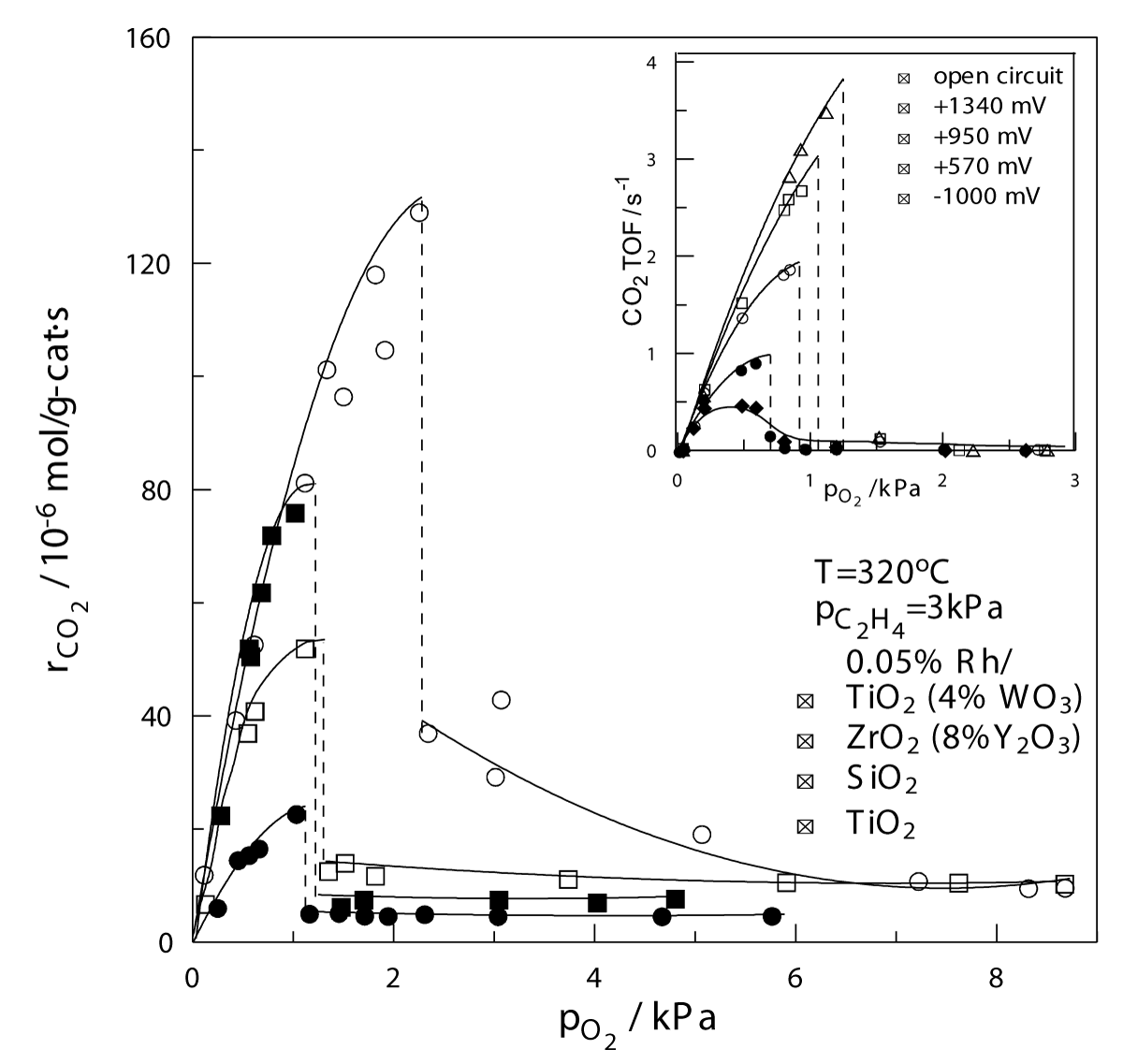
The EPOC or NEMCA phenomenon is closely related to the phenomenon of Metal-Support Interactions (MSI) in classical supported nano dispersed catalysts. The MSI phenomenon refers to the different catalytic performances observed when the same metal nanoparticles are dispersed on different supports. An example is given in Figure 1. In fact, in recent years it has become obvious that EPOC and MSI are operationally different but functionally identical phenomena and EPOC has been used to optimize the design and composition of commercial supported catalysts.
Figure 1: EPOC (inset) and MSI: Effect of pO2 on the rate of C2H4 oxidation on Rh supported on five supports of increasing work function Φ. Catalyst loading 0.05wt%. Inset: Electrochemical promotion of an Rh catalyst film deposited on YSZ: Effect of potentiostatically imposed catalyst potential UWR on the TOF dependence pO2 at fixed pC2H24. Inset reprinted with permission.
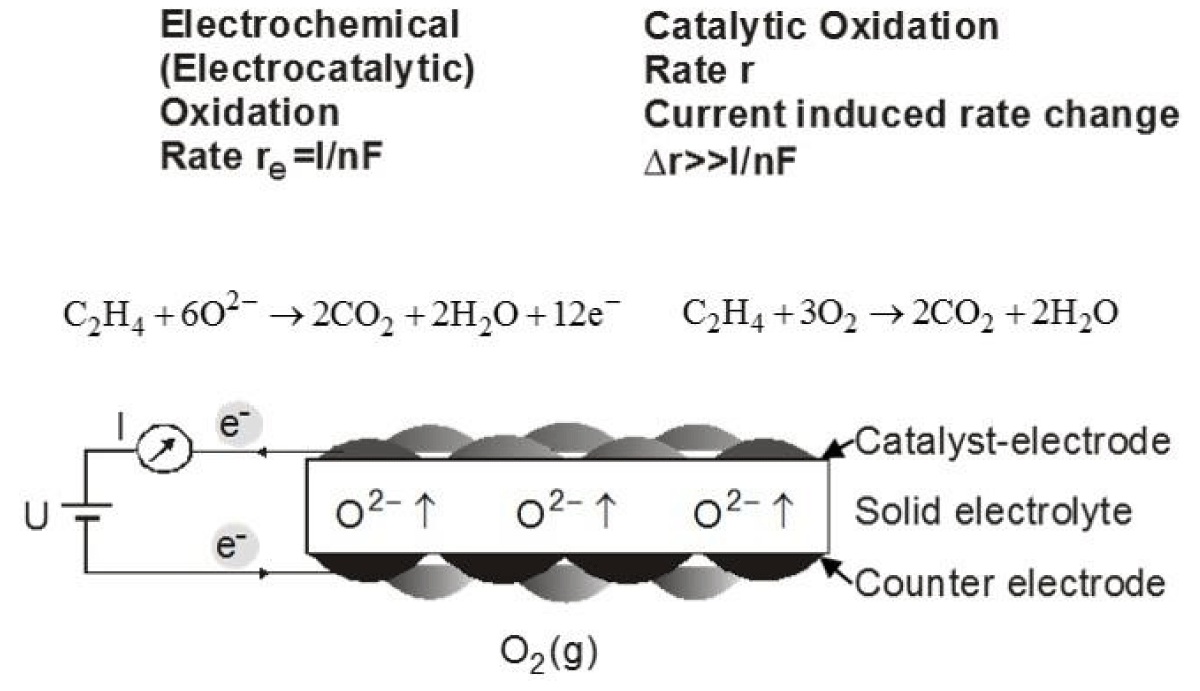
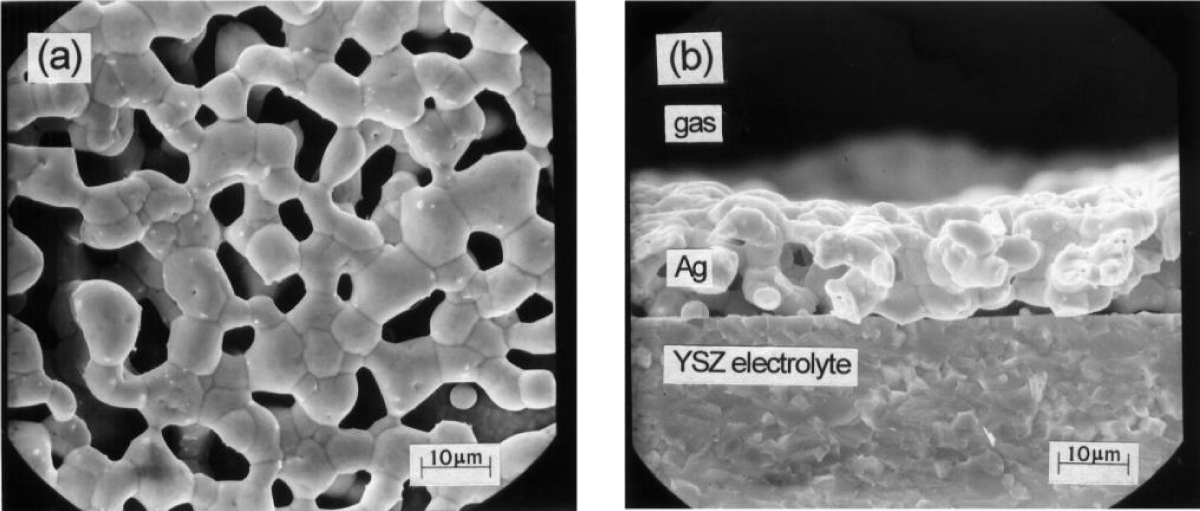
Figure 2 shows schematically the setup for EPOC studies of ethylene oxidation [1] while Figure 3 shows an SEM of an Ag-catalyst-electrode deposited on Yttria-stabilized-ZrO2 (YSZ), an O2- conducting solid electrolyte [1-4].
Figure 2: Experimental setup used in NEMCA experiments.
Figure 3: Scanning electron micrographs of an Ag catalyst-electrode deposited on YSZ and used for NEMCA studies (a) Top view (b) Cross section of the Ag/YSZ interface. Reprinted with permission from Academic Press.
Two parameters are commonly used to describe the magnitude of the Electrochemical Promotion of Catalytic reactions (EPOC):
The Faradaic efficiency, Λ, defined from
(1)
Where ∆r is the induced change in catalytic reaction rate (in mol O/s) and I denote the applied current. A catalytic reaction is said to exhibit Electrochemical Promotion (EPOC) when Λ > 1. Faradaic efficiency Λ values up to 105 has been measured [2,3].
The rate enhancement ratio, ρ, defined from
(2)
Where ro is the open-circuit catalytic rate, i.e. the rate before current application; Rate enhancement ratio, ρ, value up to 105 have been measured [1-8].
A catalytic reaction is termed electrophobic if Ʌ > 0, i.e. if the rate increases with positive applied current, i.e. with increasing catalyst potential, VWR, with respect to a reference electrode, i.e. . Examples are given in Figures 1,4. It is termed electrophilic when the opposite holds, i.e. Ʌ < 0, thus . In electrophobic reactions, the electron acceptor reactant (e.g. O2 or O2-) is more strongly adsorbed on the catalyst surface than the electron donor reactant, e.g. H or C2H4. The opposite holds for electrophilic reactions.
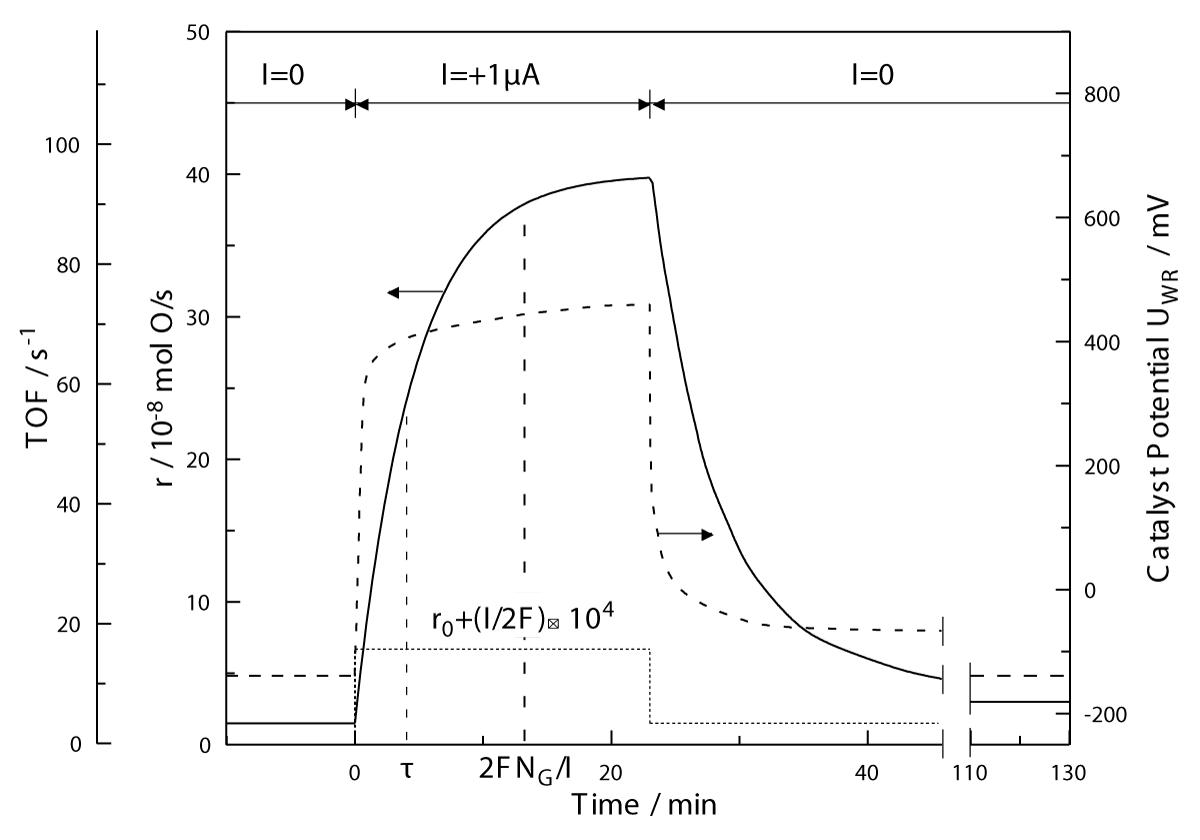
Figure 4: Rate and catalyst potential response to step changes in applied current during C2H4 oxidation on Pt deposited on YSZ, an O2- conductor. T = 370 oC, pO2= 4.6 kPa, pC2H4= 0.36 kPa. The catalytic rate increase, Δr, is 25 times larger than the rate before the current application, r0, and 74000 times larger than the rate I/2F, of O2- supply to the catalyst. NG is the Pt catalyst surface area, in mol Pt, and TOF is the catalytic turnover frequency (mol O reacting per surface Pt mol per s). Reprinted with permission from Academic Press [3].
The EPOC phenomenon is closely related to classical (chemical) promotion and to the phenomenon of Metal-Support Interactions (MSI).
EPOC has been described in several books and review articles [7,8] by more than 20 catalytic groups [8] and for more than 200 catalytic reactions [8]. It is now well established that EPOC is due to an electrochemically controlled, via electrical potential application, reversible migration of promoting ionic species from the solid electrolyte support to the gas-exposed catalyst surface.
These promoting species (i.e. Oδ- in the case of O2- conduction supports such as YSZ, Na,sup>δ+
in the case of Na+ conducting supports) migrate on the gas-exposed, catalytically active catalyst surface and affect via lateral attractive or repulsive electrostatic interactions the coadsorbed reactants and products.Figure 4 shows a typical galvanostatic (constant current) transient of C2H4 oxidation on Pt/YSZ.
Of central importance for the understanding of the mechanism of EPOC was the realization that ions from the ionically conducting supports can provide via electrical potential application spillover ions (Oδ-, Naδ+,….) which establish an effective double layer on the gas exposed catalyst surface. This double layer formed via spillover ions affects the work function of the catalyst surface and thus the binding energies of coadsorbed reactants, intermediates, and products [6-8].
Catalytic reactions were thus also soon directly grouped in four categories on the basis of the sign of the rate change observed upon positive potential application. Nucleophilic (or electrophobic), electrophilic, volcano-type, and inverted volcano type [6-8]. Simple analytical expressions were derived describing the rate vs work function dependence in excellent agreement with the experiment [6-8].
It was soon realized that the same rules also applied to classical ex-situ promotion and to the phenomenon of MSI and that therefore promotion, electrochemical promotion, and metal-support interactions are fundamentally identical phenomena and only operationally different.
Simple expressions were also derived for the time constants of galvanostatic, i.e. fixed applied current, rate transients, and the first multichannel and multiplate EPOC units were designed and built. At the same time, aqueous NEMCA was demonstrated with Pt catalyst electrodes in aqueous alkaline solutions [5].
Current research efforts focus on two main directions: First, the design and testing of larger, simpler, and more efficient multiplate electropromoted units, and second Electrochemical Promotion of monodispersed catalysts.
The latter is more difficult since the particles of fully supported nano dispersed catalysts are already at least partially promoted via ion migration from the support to the surface of the nanoparticles. Such EPOC transients are often quite complex, exhibiting rate and potential spikes. The successful interpretation of such transients and concomitating electroporation of such catalysts remains both challenging and promising and could lead to practical applications.
- Stoukides M, Vayenas CG. The effect of Electrochemical Oxygen Pumping on the Rate and Selectivity of Ethylene Oxidation on Polycrystalline Silver. J. Catal. 1981; 70(1): 137-146. https://doi.org/10.1016/0021-9517(81)90323-7
- Bebelis S, Vayenas CG. Non-Faradaic Electrochemical Modification of Catalytic Activity: 1. The case of Ethylene Oxidation on Pt. J Catal 1989; 118:125-146. https://doi.org/10.1016/0021-9517(89)90306-0
- Vayenas CG, Bebelis S, Ladas S. Dependence of Catalytic Rates on Catalyst Work Function. Nature. 1990; 343(6259): 625-627. https://doi.org/10.1038/343625a0
- Vayenas CG, Bebelis S, Neophytides S, Yentekakis IV. Non-Faradaic Electrochemical Modification of Catalytic Activity in Solid Electrolyte Cells. Applied Physics (A). 1989; 49: 95-103. https://doi.org/10.1007/BF00615471
- Neophytides S, Tsiplakides D, Jaksic M, Stonehart P, Vayenas CG. Electrochemical Enhancement of a Catalytic Reaction in Aqueous Solution. Nature. 1994; 370: 45-47. https://doi.org/10.1038/370045a0
- Vayenas CG, Brosda S, Pliangos C. Rules and mathematical modeling of electrochemical and chemical promotion: 1. Reaction classification and promotional rules. J. Catal. 2001; 203(2): 329-350. https://doi.org/10.1006/jcat.2001.3348
- Vayenas CG, Bebelis S, Pliangos C, Brosda S, Tsiplakides D. Electrochemical Activation of Catalysis. Kluwer Academic / Plenum Publishers, New York. 2001.
- Vernoux P, Lizarraga L, Tsampas MN, Sapountzi FM, De Lucas-Consuegra A, Valverde JL, Souentie S, Vayenas CG, Tsiplakides D, Balomenou S, Baranova EA. Ionically conducting ceramics as active catalyst supports. Chem Rev. 2013 Oct 9;113(10):8192-260. doi: 10.1021/cr4000336. Epub 2013 Jul 5. PMID: 23826949.