More Information
Submitted: October 25, 2022 | Approved: October 31, 2022 | Published: November 01, 2022
How to cite this article: Tajouri T. Interface of polymers grafted on silica: Organization of the interfacial layer in presence and absence of solvent. Ann Adv Chem. 2022; 6: 060-062.
DOI: 10.29328/journal.aac.1001034
Copyright License: © 2022 Tajouri T. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Interfaces; NMR; Grafted polymer
Interface of polymers grafted on silica: Organization of the interfacial layer in presence and absence of solvent
Tajouri T*
University of Tunis, IPEIT, Materials and Fluids/LR19ES03, Laboratory 2, rue Jawher Let Nahrou – Montfleury, 1008, Tunis, Tunisia
*Address for Correspondence: Tajouri T, University of Tunis, IPEIT, Materials and Fluids/LR19ES03, Laboratory 2, rue Jawher Let Nahrou – Montfleury, 1008 Tunis, Tunisia, Email: [email protected]
The 1H NMR technique is used to study the behaviour of the poly (ethylene oxide) (PEO) chains grafted chemically on silica in the presence or not of solvent. A noticeable influence of the different physicochemical parameters on the conformation of the grafted macromolecules is evinced. Different models are proposed for different lengths of grafted polymer chains on the surface in the absence and presence of a solvent. Without solvent, the macromolecules lie very flat and the layer is more organized. In the presence of a solvent, the chains spread out and adopt a more extended conformation and the local concentration of monomers decreases.
To modify the interaction between the solid and the surrounding medium one of the ways is to graft polymers at the interface. The interfacial layer can act as a coupling agent a screening factor and enhance the properties of the interface. Polymer chains grafted into solid surfaces are fundamental in many applications in colloid and surface science, as well as in biology [1].
Such interfaces are used in colloid stabilization and chromatography [2], where this interfacial layer controls selectivity. Similar interfaces are also found in composites, for example in rubber reinforcement, where the manufacturing of materials with interesting physical and chemical properties, like tires, requires a good mixing between the filler and the matrix. In this case, the polymer at the interface acts as a coupling agent [3].
The configuration and the properties of the polymer chain should depend essentially on the polymer surface density [4,5] polymer length and the nature of the solid surface. Indeed one of the fundamental investigations concerning a polymer grafted on a solid surface is the determination of the disposition of the macromolecules at the surface and its corresponding mobility. Different methods and approaches have been used to study these systems and to understand their behaviour, whose results should finally give a coherent overall picture of their structure [6,7]. Only limited data concerning the chain dynamics are available.
For this study we choose poly (ethylene oxide) (PEO); it is a simple polymer that can be used as a model system since all the CH2 are chemically identical (with the exception of the terminal group) and the 1H and 13C NMR spectrum of the polymer has only one simple line.
The strong PEO/silica interactions cause the polymer to adopt a flat conformation, independently of the degree of grafting, by spreading on the solid surface to occupy the maximum area in the absence of solvent [8].
In this paper, we describe the behaviour of PEO with different lengths of the grafted polymer chains on the surface in the absence of and presence of a solvent.
Silica: the silica used was pyrogenic Aerosil A300 (from Degussa), prepared by a thermal process. This powder is essentially not porous and consists of large aggregates of small spheres, from 7 nm to 12 nm in diameter. Its specific area, measured by nitrogen gas adsorption, is ca. 300 m2g-1. This material was selected because it has a high degree of chemical purity and it is not porous at a molecular scale [8]. In fact, at the scale of the silica surface was a characteristic length of macromolecular chains, and their radius of gyration was reasonably planar.
Polymer: the polymer was poly (ethylene oxide) (PEO). Polymer lengths and polydispersities were controlled by gel permeation chromatography. The oxygen in the backbone makes it very flexible. In NMR this polymer is very convenient because there is only one existing chemical shift as well for 1H than for 13C.
Solvent: as a solvent, deuterated benzene is used in order not to produce a proton NMR signal and to decrease the dipolar interaction between the nuclei of benzene and the nuclei of polymer.
MR technique
Experimental 1H and 13C measurements were carried out on a Bruker CXP 300 Fourier transform NMR spectrometer operating at 300 MHz (1H) and 75 MHz (13C).
Experimental measurements of T1 (1H) were carried out by using the inversion-recovery pulse sequence (π − τ − π/2).
The 13C NMR measurements were performed using the cross-polarisation and magic angle spinning methods.
Influence of the polymer chain length on the relaxation times
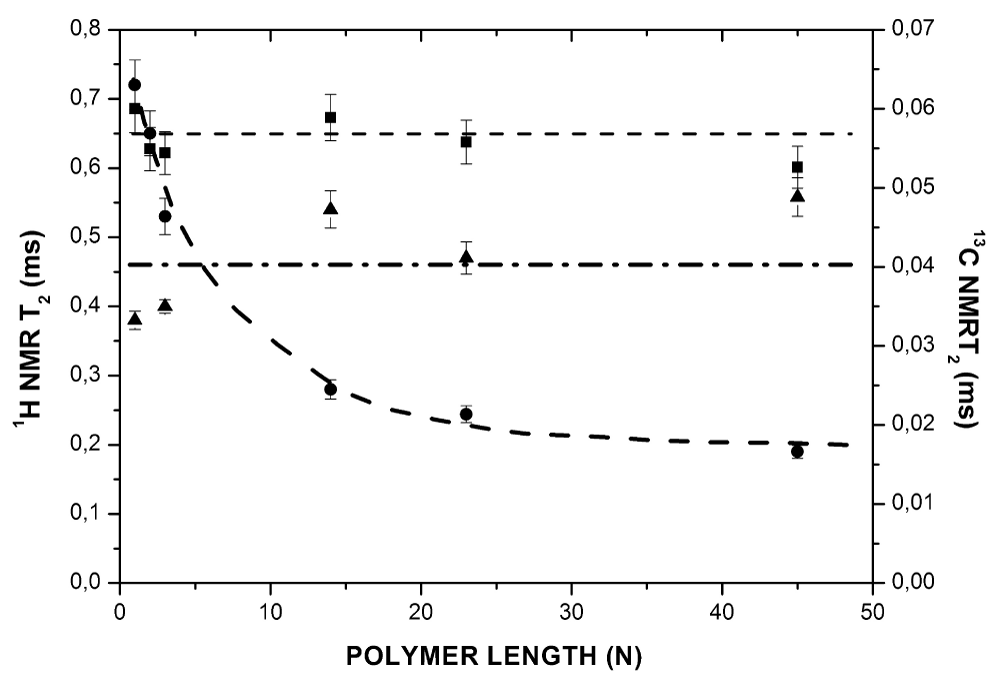
To study the evolution of the surface layer of the grafted polymers with the polymer length, samples ranging from N = 1 for the monomer of ethylene oxide to N = 45 for PEO were used. The results of the measurements of 1H and 13C spin-spin relaxation times T2, as a function of the degree of polymerization, denoted N, are shown in Figure 1.
Figure 1: Evolution of the spin-spin relaxation time (T2) as a function of polymer length (N) for grafted PEO: 1H NMR without solvent (), 1H NMR with solvent (deuterated benzene) () and 13C NMR without solvent (). Data obtained from 1H and 13C NMR spectroscopy (300 MHz).
Without solvent, the 1H spin-spin relaxation time, T2, decreased with increasing polymer length. However, when the compound was immersed in deuterated benzene, T2 increased, varying from 0,38 ms to 0,55 ms. The evolution of the 13C spin-spin relaxation time, T2, for the sample without solvent appeared to be completely independent of the polymer length.
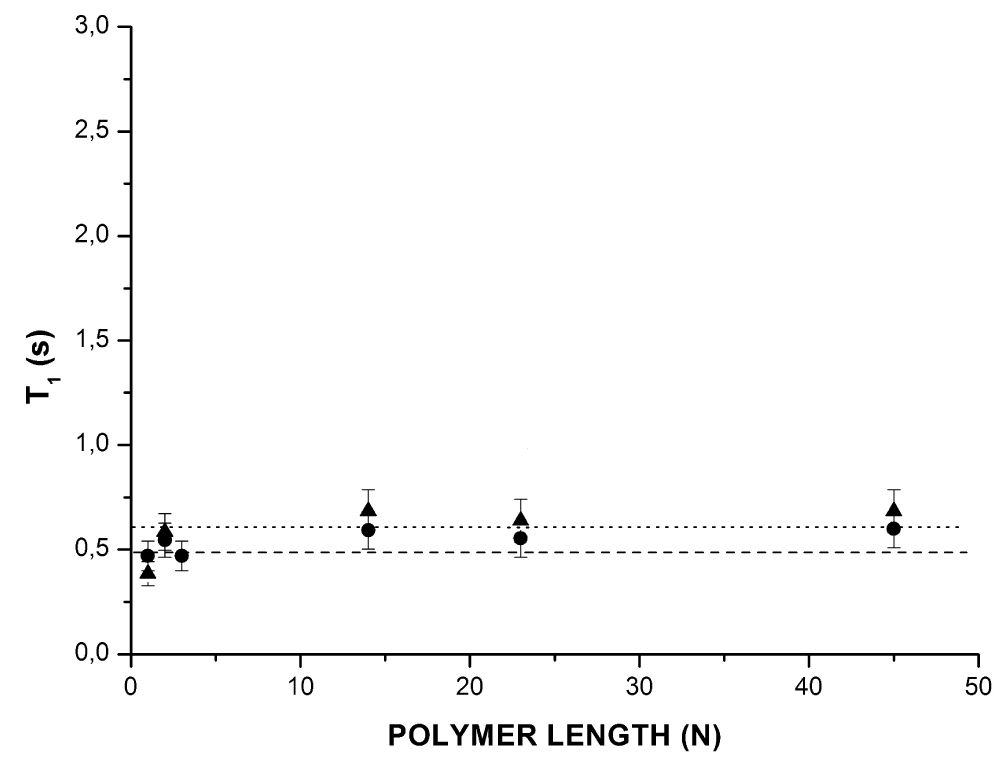
Figure 2 shows the evolution of the spin-lattice relaxation times, T1, as a function of polymer length N. The spin-lattice relaxation time did not depend on the length of the polymer, both in the presence and in the absence of solvent. It has previously been shown that the 1H spin-spin relaxation time, T2, for all polymers is sensitive to the slow processes of motion of the monomer units (inter-monomer units contribution) and the local concentration of the monomer units [9]. On the other hand, the spin-lattice relaxation time, T1, describes the fast movements occurring at frequencies close to the Larmor frequencies. Thus, T1 is sensitive to the characteristic motion frequencies of the order of the Larmor frequency (fast processes of motion) and is related to the local motion of the monomer units rather than large-scale segmental motions. Finally, the 13C spin-spin relaxation time T2 (Figure 1) is primarily sensitive to the monomer units mobility; the 13C‑1H interaction is important only inside the monomer units [10]. Indeed, the dipolar interaction of 13C nuclei with neighboring nearby 1H was more intense, as would be expected, than that with other more distant 1H. It was also very weak or even zero with the other nuclei because of their low natural abundance.
Figure 2: Evolution of the spin-lattice relaxation times, T1, as a function of polymer length (N) for grafted poly (ethylene oxide): without solvent () and with solvent (deuterated benzene) (). Data obtained from 1H NMR spectroscopy (300 MHz).
By 1H NMR we observed that the spin-spin relaxation time, T2 decreased with increasing polymer length, whereas the T2 did not change in the 13C NMR, as shown in Figure 1. Since the essential difference between 1H and 13C NMR is the sensitivity to the inter-monomer units dipolar interaction, which is only observed by 1H NMR, it implies that this interaction increased with the polymer length.
organization of the interfacial layer
As was mentioned above, the slow mode of the segment motion which governs the spin-spin relaxation time of 13C [11] does not change with polymer length. When the sample is immersed in deuterated benzene, the polymer length effect disappears because the local monomer unit concentration decreases, i.e. the dynamic fluctuations of the segment‑ distance are higher and may average the inter-monomer units’ dipolar interaction. In this case, it is only a fluctuating effect that gives a relaxation mechanism superposed to the intra-monomer units spin‑ process. More precisely the Hamiltonian, which describes the dipolar interaction, can be written as mentioned below [12,13]:
is the contribution of all the protons in a monomer unit. is the dipolar interaction between the nuclei of different monomer units.
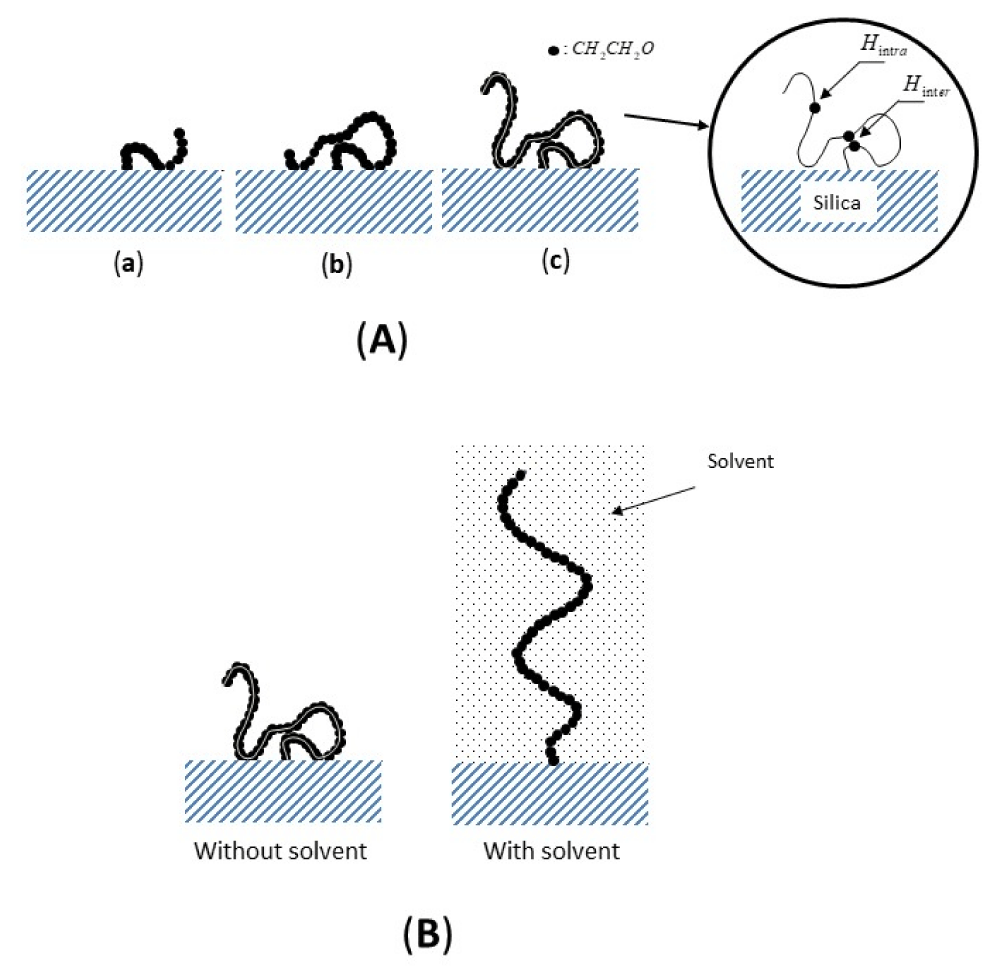
Because of the very slow and anisotropic reorientation process of one monomer unit relative to the other in the absence of solvent, the dipolar interaction Hinter between the nuclei about different segments but close in space is not completely averaged in a time characteristic of the evolution to the equilibrium of the transverse magnetization giving nonzero average dipolar spin coupling. The overall picture that thus emerges from the data can be illustrated by the schematic representations of the organization of the grafted layers given in Figure 3. In the absence of solvent (Figure 3A), as the length of the polymer chain increases, the mobile segments, far from the surface, form loops and tails while the other, less mobile ones are adsorbed on the surface.
In the presence of solvents (Figure 3B), the chains adopt a more extended conformation, destroying any loops and trains.
Figure 3: Schematic representation of the chain at the interface: (A) Without solvent for different polymer length (N): (a) N = 15, (b) N = 30, (c) N = 45; (B) With solvent for polymer length N = 45. Inset: contribution to the dipolar interactions, inter and intra (Hintra and Hinter) in a chain of poly (ethylene oxide) grafted on silica. () represents a monomer unit.
The behavior of PEO grafted on silica has been studied by 1H and 13C NMR. The analysis of relaxation times allows describing the segmental motion for the various PEO chain lengths. Dipolar interactions were not completely averaged by the reorientation motion due to the local concentration of monomer units grafted on the silica surfaces.
A noticeable influence of the length of polymers parameter (N: number of monomer units) on the dynamics and the conformation of the grafted macromolecules is evinced.
In the absence of solvent, the surface is very attractive, and the chains adopt a flat conformation and form loops and tails far from the surface, In the presence of solvents the chains adopt more extended configurations which are perpendicular to the surface destroying any loops and trains.
- Advincula RC, Brittain WJ, Caster KC, Ruhe J. Polymer Brushes. Wiley-VCH, Weinheim. 2004.
- Buijten J, Blumberg L, Markides K, Wannmann T, Chromatogr J. 1983; 268:367
- Bouty A, Petitjean L, Degrandcourt C, Gummel J, Kwaśniewski P, Meneau F, Boué F, Couty M, Jestin J. Nanofiller Structure and Reinforcement in Model Silica/Rubber Composites: A Quantitative Correlation Driven by Interfacial Agents. Macromolecules. 2014; 47:5365.
- Hommel H. (ed) Polymers, surfaces:a versatile combination. Trivandrum, India. Research Signpost. 1998.
- De Gennes PG. Adv. Colloid Interface Sc. 1987; 27:189
- Kato K, Uchida E, Kang ET, Uyama Y, Ikada Y, Prog. Polym. Sci. 2003; 28:209
- Metin B, Blum F. Segmental Dynamics in Poly(methyl acrylate) on Silica: Effect of Surface Treatment. Langmuir. 2009; 26:5226
- Tajouri T, Bouchriha H. Nuclear magnetic resonance relaxation study of the organization of layers of poly(ethylene oxide) chains grafted on silica. Polymer. 1996; 37:185.
- Tajouri T, Hommel H. Dynamics and conformations of PEO chains chemically bonded on silica: comparison between 1H and 2H NMR. Magn Reson Chem. 2007 Jun;45(6):488-95. doi: 10.1002/mrc.1997. PMID: 17431859.
- Andrew ER, Phil. Trans. R Soc. Lond. A 1981; 292:505
- Cohen-Addad JP. Effect of the anisotropic chain motion in molten polymers: The solidlike contribution of the nonzero average dipolar coupling to NMR signals. Theoretical description. Chem J Phys. 1974; 60:2440
- Tajouri T, Ben Mariem MA, Hommel H. “Pseudo solid” nuclear spin echo study of poly (ethylene oxide) grafted on silica. Comparison with magic-angle spinning technique results. Concepts Magn. Reson. 2015; 44:306
- Tajouri T, Bouchriha H. Study by 1H NMR of the concentration of monomer-units on interface polymer grafted on silica using magic angle spinning and relaxation techniques. J Colloid Interface Sci. 2002 Aug 15;252(2):450-5. doi: 10.1006/jcis.2002.8507. PMID: 16290811.