More Information
Submitted: December 28, 2022 | Approved: January 30, 2023 | Published: January 31, 2023
How to cite this article: Alrbaihat MR, Abu-Afifeh Q. Eco-friendly microplastic removal through physical and chemical techniques: a review. Ann Adv Chem. 2023; 7: 0014-024.
DOI: 10.29328/journal.aac.1001038
Copyright License: © 2023 Alrbaihat MR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Microplastics; Physical methods; Removal techniques; Membrane-based; Chemical treatments
Eco-friendly microplastic removal through physical and chemical techniques: a review
Mohammad R Alrbaihat1* and Qusay Abu-Afifeh2
1Department of Land, Water and Environment, The University of Jordan, Amman, Jordan
2Teacher Training Institute, Ministry of Education, Dubai, 3962, United Arab Emirates
*Address for Correspondence: Mohammad R Alrbaihat, Department of Land, Water and Environment, the University of Jordan, Amman, Jordan, Email: [email protected]
A growing number of synthetic plastics derived from fossil fuels are produced, and improper plastic waste management has caused a lot of pollution problems. There are many microplastics in the environment, and they disintegrate slowly in soil and water. The properties of microplastics (MPs) include long residence times, high stability, high fragmentation potential, and the ability to adsorb other contaminants. Invertebrates and planktonic organisms are easily able to accumulate microplastics in aquatic species. Therefore, microplastics (MPs) must be removed from the water and other media. This paper aims to review the occurrence, raw polymers and additives, and remediation methods for removing microplastics from the environment. Several methods are available for removing contaminants, including sorption, filtration and chemical treatments. Various removal methods are discussed along with their methods, efficiency and advantages.
Globally, plastic production increased dramatically from 1950 to 2015 to improve human quality of life [1]. As a result, plastic pollution has increased worldwide, posing a threat to environmental health [1-3], approximately 0.59 × 109 particles of microplastic are released by sewage treatment plants each year into aquatic ecosystems [1]. The term microplastic refers to plastics smaller than 5 mm in size, formed when many plastic-based products are exfoliated and degraded into ecosystems [4]. Marine sediments [5], urban and rural areas [6], freshwaters [7], and seawaters [8] have all been reported to contain microplastics. According to most studies, microplastics accumulate in aquatic environments, increasing the exposure of living organisms to microplastics and the degradation products created by them [9,10].
Generally, microplastics (MPs) can be divided into primary microplastics, which are raw materials used in the manufacturing of household and personal care products, whereas secondary microplastics come from discarded materials or remnants of production, which are materials that develop through physical, chemical, and biological degradation in the environment [1,11]. Microplastics are a concern for environmental scientists due to their long-term durability and their ability to easily travel between different habitats (Figure 1) [1].
Figure 1: Common Microplastics sources (modified from [1]).
The most common raw polymers are polyethylene terephthalate (PET), polyurethane (PU), polystyrene (PS), polyvinyl chloride (PVC), polypropylene (PP), polyesters, polyethylene (PE) and polyamides (PA, nylon). Microplastics are ubiquitous because of poor plastic waste management [12,13].
Chronic exposure to microplastics is found to be toxic, but there is no evidence that they cause acute fatality [12,13]. Chemical structure, additives used during polymerization, and how they are linked during polymerization control toxicity of microplastics [13,14]. Microplastics, such as polystyrene, are capable of crossing into the bloodstream and disrupting the reproductive process of marine filter feeders [1,8].
We discussed microplastic removal during this review. The sources of microplastic additives are discussed along with their occurrence, followed by a review of removal methods. Physical methods of removal include sorption and filtration, as well as chemical processes based on chemical phenomena.
Toxicity of microplastics
The potential toxicity of microplastics arises from un-reacted monomers, oligomers, and chemical additives leaked from the plastic in the long run [1]. Types of microplastic toxicity are depicted in Table 1.
| Table 1: Kinds of microplastic toxicity. | ||
| Toxicity Type | Proposed effects | Ref |
| Structure-based toxicity | Ability to migrate from food packaging materials. | [15] |
| Potentially absorbed residuals (i.e., Polystyrene bisphenol resins) by living tissues. | [16] | |
| Chemical additives are used during polymer manufacturing (i.e., phthalates from baby bottles) that enhance anomalous embryonic development. | [17] | |
| Chemicals released from plastics, like benzene, toluene, ethylbenzene, styrene, etc., may also cause chronic health effects. | [18] | |
| Physicochemical toxicity | The large surface area/volume ratio of microplastics causes them to cause damage, that effect aquatic animals and then carries them to other habitats. | [19] |
| Significant liver and brain tissue changes exposed to low-density polyethylene glycol microplastics containing phenanthrene. | [20] | |
| An adverse effect of microplastics on algae photosynthesis | [21] | |
| Ingested microplastics can also be toxic and absorbent depending on the shape and texture. | [22] | |
| Microorganism toxicity | Pathogenic bacteria on some polyethylene, polypropylene, etc. may cause health impacts due to the micro-bacterial assemblages found in microplastics. | [23] |
| Low concentrations of airborne microplastics in the air can cause cardiovascular diseases, respiratory diseases, and interstitial lung diseases. | [24] | |
Materials, polymers and additives associated with microplastics
Microplastic chemical additives: Polyethylene, poly-propylene, and polystyrene are the most common polymer components of primary microplastics, depending on the type of products being manufactured; while polyester, acrylic, and polyamide are the most common polymer components of secondary microplastics, forming fibers in the environment [25]. Polyethylene terephthalate and polypropylene were major types of microplastic, e.g., in Wuhan’s inland freshwaters. 1650.0 ± 639.1 and 8925 ± 1591 numbers/m3 were the major types here. The strongest type of microplastic has also been found to be low-density polyethylene [18].
Chemical additives such as bisphenol A, polybrominated diphenyl ethers, and phthalates are commonly found in raw plastics to enhance plasticity [26]. In addition to causing endocrine disruption, these additives may also be toxic. Such plasticizers are present in wide ranges in the plastic debris of remote and urban beaches: Bisphenol A is found in up to 35 ng/g of plastic debris on remote beaches, polybrominated diphenyl ether reaches up to 9900 ng/g on urban beaches, and phthalates are found in up to 3940 ng/g of plastic debris on urban beaches [6]. Most microplastic polymers have been detected with these plastic additives [25]. Additionally, researchers reported that silicone and polycarbonate microplastics could leach bisphenol A and nonylphenol [27]. There has also been a report of such chemicals accumulating in the human body through biological processes [28]. Microplastic exposure via food is one of the most alarming routes for humans [29], where the adverse effects of the chemical additives and mechanism of entry into the body are still under investigation. Thus, finding strategies for reducing the presence of microplastics in the environment must be a key objective. There have been reports on identifying the sources and occurrence of microplastics, their fate, methods for detection, and their environmental effects; however, to date, very few research and review papers have discussed how microplastics can be removed from a a contaminated environment.
Sources and incidences of microplastics: The transport phenomena involved in transporting microplastics such as wind and ocean currents contribute to their widespread presence in coastal regions and aquatic ecosystems worldwide [30]. Plastic pellets or powders used for air blasting are among the primary sources of polymers in household sewage discharge, including polymers from cosmetics and cleaning products [25]. In a secondary source of microplastics, the progressive fragmentation of larger plastic items in the environment (e.g., via mechanical degradation and UV exposure), contributes to the entry of substantial amounts into the environment through mechanical degradation and UV exposure [31]. By increasing plastic debris availability for ingestion by a wide range of organisms, we highlight the possibility of environmental hazards increasing [32].
It is also common for wastewater treatment facilities to release microplastics [33,34]. Microplastics often bypass wastewater treatment and enter and accumulate in aquatic environments, even when larger plastic particles are effectively removed during wastewater treatment [35].
The treatment of wastewater frequently bypasses the removal of microplastic particles, which accumulate in aquatic environments despite the convenience of being removed from larger plastic particles [35]. There are many water treatment plants located near oceans and seawater, causing microplastics to be released into the environment. According to data from mainland China, out of 3340 wastewater plants, almost 1873 (58%) have treatment capacities of 78 × 106 m3/day and are located along coasts where effluent can be discharged directly or indirectly into aquatic ecosystems [36]. To address this problem, many researchers are researching how microplastics are eliminated from water treatment plants by investigating their fate, occurrence, detection, and removal of these particles [37,38].
Removal of microplastics using physical methods: This study reviewed various physical techniques that are efficiently applied for the removal of microplastics (MPs) from treatment water.
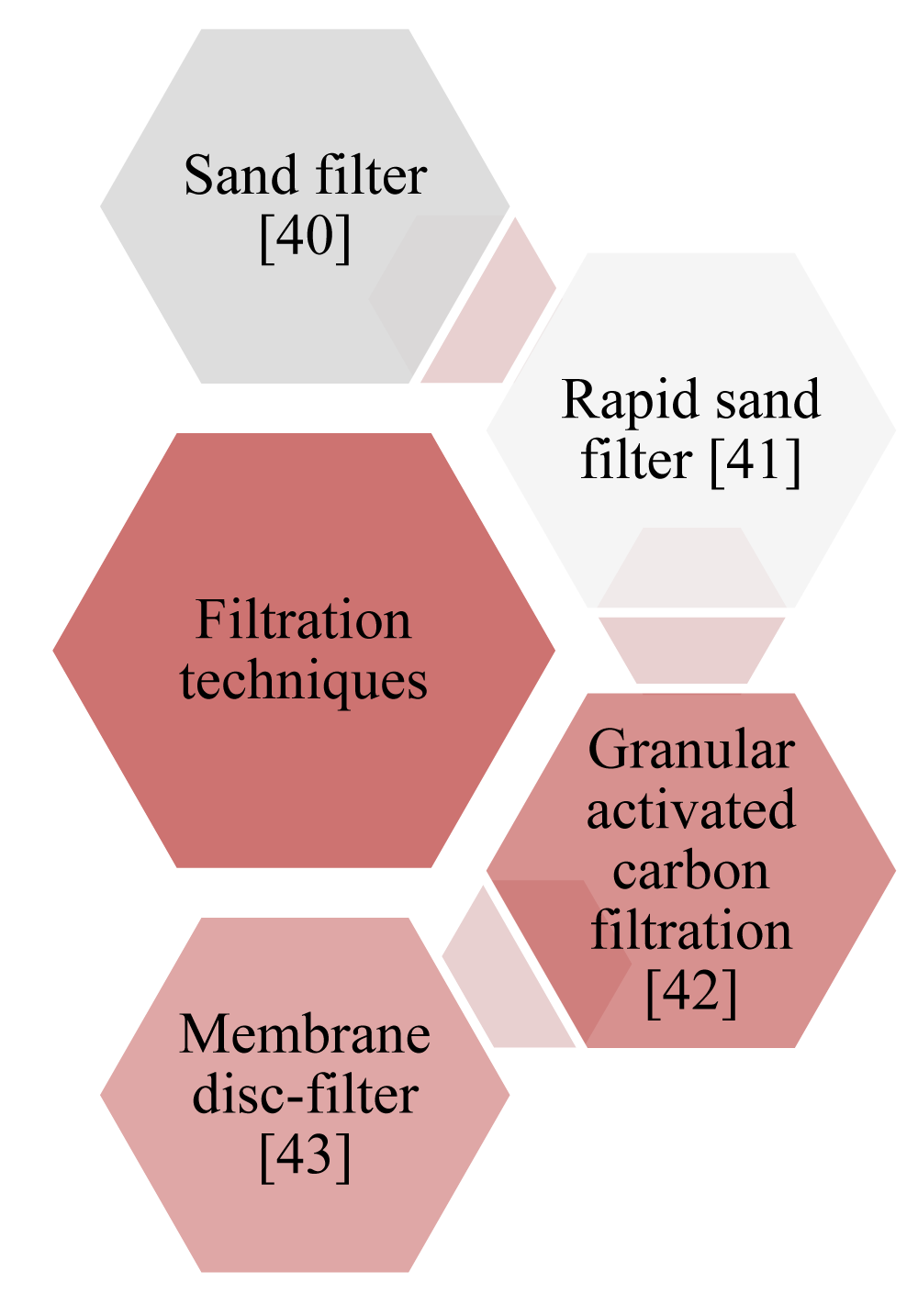
Advanced filtration technology for microplastic removal: Several filtration techniques are utilized for MPs removing classified under physical methods as shown in Scheme 1 [39].
Scheme 1: Filtration techniques for microplastic removal [40-43].
Recently, Lares, et al. (2018) applied a combination of membrane bioreactors/conventional activated sludge to investigate the performance of a municipal wastewater treatment plant. They sampled every 2 weeks for 3 months [44]. Finland’s Mikkeli city center is home to a municipal water treatment plant that was used to collect samples of wastewater. As long as the conventional activated sludge system contains an aeration tank, where the wastewater is mixed with air to activate micro-organisms, and a sedimentation tank, where the treated wastewater is separated from the sludge, for subsequent biological treatment and secondary purification, the efficiency should also be improved [44].
It is also possible to use a conventional activated sludge system is also expected to be improved by the addition of aeration tanks for mixing water with air and sedimentation tanks for separating the sludge from the treated wastewater for biological degradation and secondary purification [45,46]. Membrane bioreactors were significantly better at removing microplastics (99.4%) than conventional activated sludge treatment systems (98.3%). It was estimated that in the water effluent of the former system, the microplastic concentration was 0.4 ± 0.1 MP/L, which was lower than that found in the water effluent of the latter system (1.0 ± 0.4 MP/L). Moreover, the study authors pointed out that using slightly different processing steps and wastewater samples in their study may have contributed to the narrow range of final microplastic concentrations [35,47].
By observing the dimensional changes, abundance, shape, and color occurring during the removal steps, researchers in Changzhou, China, evaluated microplastic removal efficiency at their wastewater treatment plants [48]. Almost all plants using a combination of floating and sedimentation tanks, as well as filtration processes eliminated over 90% of microplastics from the influents. The final removal of microplastics reached 97.15%. Depending on the volume of processing daily, the type of raw water, and the type of treatment process, the removal efficiency may vary considerably. This was my previous report, which reported less abundance of large microplastics in the effluents [49]. Furthermore, these microplastics were mainly composed of fiber rayon and polyethylene terephthalate, as evidenced by the high removal rates [49].
A study published by Yang, et al. 2019 presented the results of a study by researchers in Beijing, China in which microplastics were removed from municipal sewage treatment plants [50]. Anoxic, aerobic and anaerobic A2O treatments were used during the initial treatment process of the influents. This included an aerated grit chamber, primary and secondary sedimentation tanks, and an aerated grit chamber. Denitrification, ultrafiltration, ozonation and ultrasound are techniques used to remove microplastics from wastewater and complete the treatment process [50].
Polyethylene terephthalate and polyester rank first in abundance in the effluent, with 42.26% and 19.1%, respectively, according to FTIR analysis. Microplastics were removed from influents with an efficiency of 58.84% following the primary treatment using aerated grit and thus 71.67% following the advanced treatment procedures. It was comparable to the efficacy of dissolved air flotation and sand filters despite the current sewage treatment plant’s 90.166% removal efficiency being significantly lower than the 99.9% average of membrane bioreactors [50,51]. The current treatment systems are not effective enough to remove microplastics from sewage treatment plants. Although these processes do not eliminate all microplastics from wastewater, they do eliminate a good percentage of them.
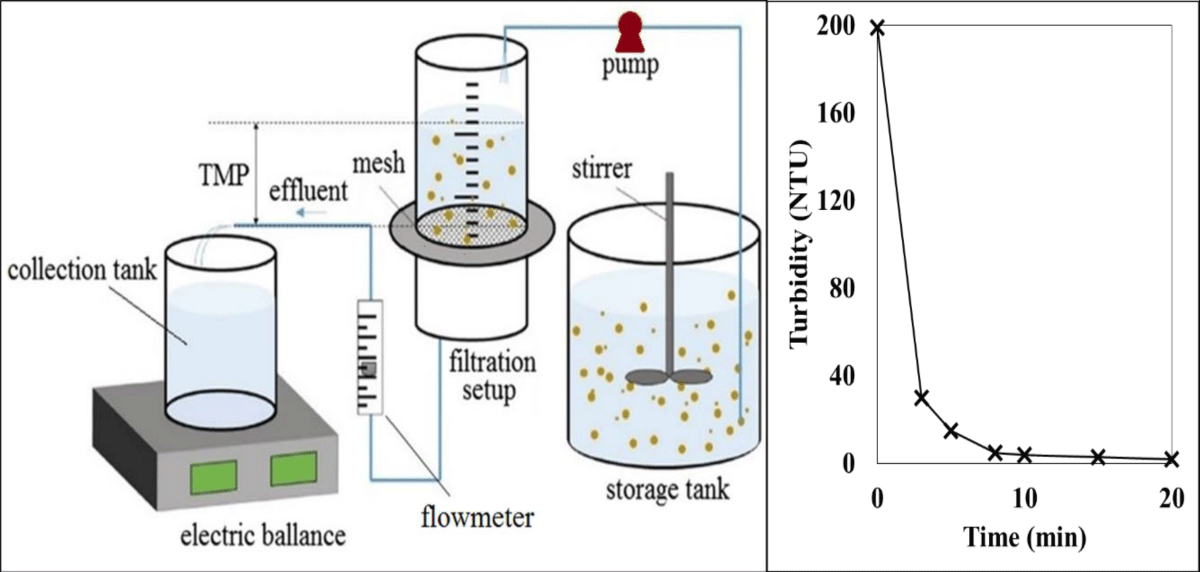
Membrane-based technology for the removal: During the study of Li, et al. (2018), dynamic membranes were used to effectively remove microplastics from synthetic wastewaters, Figure 2 shows the decrease in turbidity [39] when microplastics are removed from synthetic wastewaters. The study applied dynamic membranes for the effective removal of microplastics. Influence of flux and particle concentration during filtration of synthetic wastewater on the removal efficiency of dynamic membranes formed on a diatomite platform with 90 μm of supporting mesh.
Figure 2: Graph and set up for the dynamic membrane experiment (modified from [54]).
When artificial wastewater was filtered with a diatomite platform with a 90 μm mesh of supporting mesh, the effect of influent flux and particle concentration was determined. Microplastics were filtered to near-zero turbidity in 20 minutes by reducing the influent turbidity from 195 NTU to less than 1 for the effluent [49], [52]. Input fluxes and microplastic concentrations are both factors that facilitate membrane formation. Based on elongated polymer coatings and mesh screens, researchers have developed an efficient microplastic removal tool. According to him, the tool has good durability, can be easily fabricated from common materials, and is durable. Additionally, there are no mechanical or electrical devices with these tools [1,53].
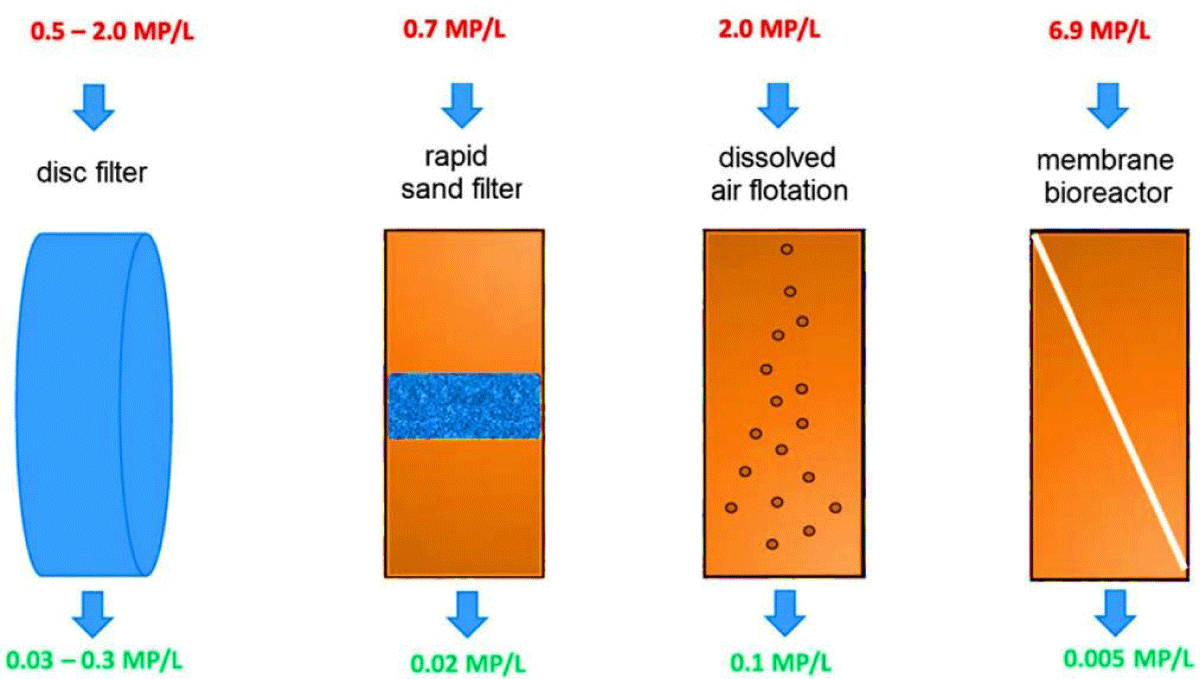
There is, however, a greater capacity for the removal of micro-sized plastics within membrane bioreactors than in simple dynamic membranes [55]. Kno- block, et al. (1994) explored the possibility of purifying a combined system by taking advantage of porous membranes along with biological processes [56]. The successful use of membrane bioreactors to remove high-level contaminants such as polymeric debris and microplastics confirms the suitability of this technology to handle complex industrial wastewater [46]. Talvitie, et al. (2017) conducted a study to examine how microplastics were removed from wastewater treatment plant effluents by using advanced end-stage technologies, including membrane bioreactors, disk filters, rapid sand filters, and dissolved air flotation as shown in (Figure 3).
Figure 3: Amount of microplastics removed with final-stage technologies, measured in microplastics per liter, MP/L (modified from [51]).
Based on their analyses, the membrane bioreactor eliminated 99.9% of microplastic particles from 6.9 to 0.005 per L of water (Table 2). Additionally, microplastics of any size, even those of 20 to 100 microns, were removed by membrane bioreactors, rapid sand filtration, and dissolved air flotation [51].
| Table 2: Average concentrations of microplastics before and after treatment with various technologies [51]. | ||||
| Treatment | Effluent type | Before (MP/L) | After (MP/L) | Removal (%) |
| Disk filter 10a | Secondary | 0.5 | 0.3 | 40.0 |
| Disk filter 20a | Secondary | 2.0 | 0.03 | 98.5 |
| Rapid sand filter | Secondary | 0.7 | 0.02 | 97.1 |
| Dissolved air flotation | Secondary | 2.0 | 0.1 | 95.0 |
| Membrane bioreactor | Primary | 6.9 | 0.005 | 99.9 |
| Each microplastic concentration is measured in microliters of effluent. The pore size is μm. | ||||
Furthermore, A significant amount of microplastic was effectively removed from influents and effluents during treatment, irrespective of the shape of the microplastic. An analysis of the samples using Fourier transform infrared spectra (FTIR) revealed a marked decrease in polymers in the final effluent by the membrane bioreactor, highlighting the enzyme’s ability to bind various chemical structures of microplastics [1,9,49,51]. Technologies based on membranes have been effective in removing microplastics from polluted aquatic ecosystems. Microplastics are removed more efficiently over durable membranes, have a large influent flux, and are both large and concentrated. Biological processes combined with porous membranes could enhance removal efficiency by 99.9%.
Algae adsorption: Because of the potential for entanglement and bioaccumulation of microplastics in aquatic environments, they are more critical than other pollutants [57,58]. Microplastics can cause multiple harmful effects as well as the death of aquatic organisms, e.g. reptiles, fishes, mammals, and birds. Since they are persistent and low-degradable, removal methods are required. Since they are persistent and poorly degradable, removal methods are necessary. Most microplastics are classified as persistent materials, but their nature and chemical structure determine how quickly they degrade. If the half-life times are lower than those determined by REACH criteria for consistency (Table 2), then these microplastics are degradable and are not hazardous to the environment [59]. It’s well known that microplastics adsorb and carry a wide range of contaminants from water on their surfaces, carrying them into nearby habitats and desorbing them [60]. Because of their high surface area to volume ratio, other contaminants are likely to adhere to them Table 3.
| Table 3: REACH Annex XIII provides a list of persistent contaminants in different media [61]. | |
| Compartment | Half-life (days) |
| Marine water | > 60 |
| Fresh or estuarine water | > 40 |
| Marine sediment | > 180 |
| Fresh or estuarine sediment | > 120 |
| Soil | > 120 |
According to Sundbaek, et al. (2018), fluorescent microplastic particles adhered well to the surface of seaweed, Fucus vesiculosus, an edible marine microalga. Microchannels within the plant cells of the sorbent limit the translocation of polystyrene microplastics into tissues due to the polystyrene microplastics’ 20 mm diameter. The results showed very high sorption (94.5%) of microplastics to seaweeds around the cut surfaces, which is attributed to the release of alginate compounds from the cutting processes [62]. Alginate acts as a gelatinous substance that can be used to improve the adhesion of polystyrene to the surface of seaweed due to its anionic character [63]. Microplastics and microalgae’s surface characteristics are influenced by surface charge in this paper and other studies about microalgae’s ability to adsorb plastic particles [64,65]. Researchers examined the adsorption of polystyrene particles of 20 to 500 nm on bicellular algae, Pseudokirchneriella subcapitata, Nolte, et al. (2017) considered positively charged rather than negatively charged microplastics for the most efficient adhesion [65].
Microplastics’ ability to bind to algae surfaces is strongly influenced by their surface charge. An anionic polysaccharide in the chemical structure of algal cells explains why positively charged microplastics tend to be absorbed more efficiently [66].
Microplastics: chemical treatments: Flotation and agglomeration processes are commonly used in wastewater treatment plants to produce larger constituent particles that are easier to separate [67]. Through the use of Fe- and Al-based salts along with other coagulants, these processes bind tiny particles by inducing uptake-complexation mechanisms that are initiated by exchanges of ligands, thus forming strong bonds between waste particles [68].
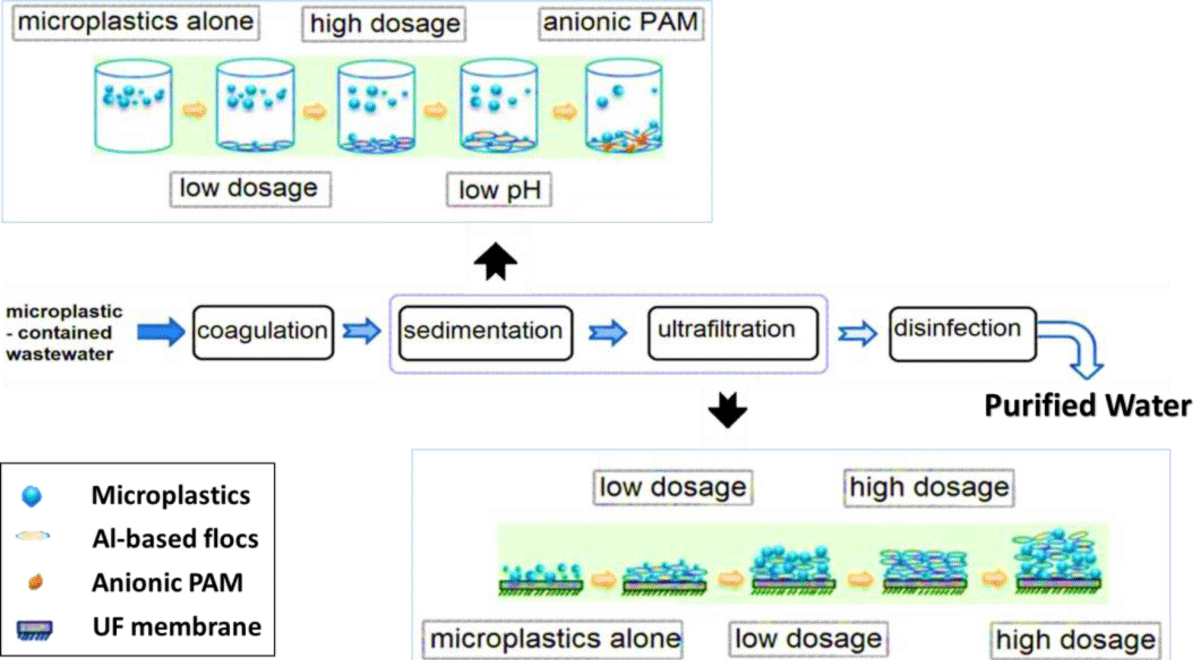
Using iron and aluminum salt coagulants and ultrafiltration, Aza-Tarazona, et al. (2019) determined the effects of anionic polyacrylamide (PAM), pH, and the formation of Al-based flocs on the removal efficiency of microplastics. The results are shown in Figure 4. The experiments were conducted with Al3+ and Fe3+ ions at different concentrations, and the results indicated that Al3+ performs better than Fe3+. Furthermore, the removal efficiency of microplastics was not significantly affected by pH in the presence of low concentrations of Al coagulant source, 0.5 mM, although removal efficiency decreased when pH was raised with small microplastics of diameter less than 0.5 mm. A high Al dosage of 5 mM did not improve the removal efficiency of small microplastics as well as it did for large particles when using polyacrylamide (PAM), an enhancing coagulant. In the presence of cationic polyacrylamide, small microplastics grow at an accelerated rate. The removal efficiency of smaller microplastics (d < 0.5 mm) was significantly enhanced when anionic polyacrylamide was used, from 25.83% without polyacrylamide to 61.19% with 15 mg/L polyacrylamides; however, the growth rate increased by just 4.27% to 18.34% for large microplastics (2 mm - 5 mm diameter) [69,70].
Figure 4: A coagulation, sedimentation, and ultrafiltration process for removing microplastics (modified from [48]).
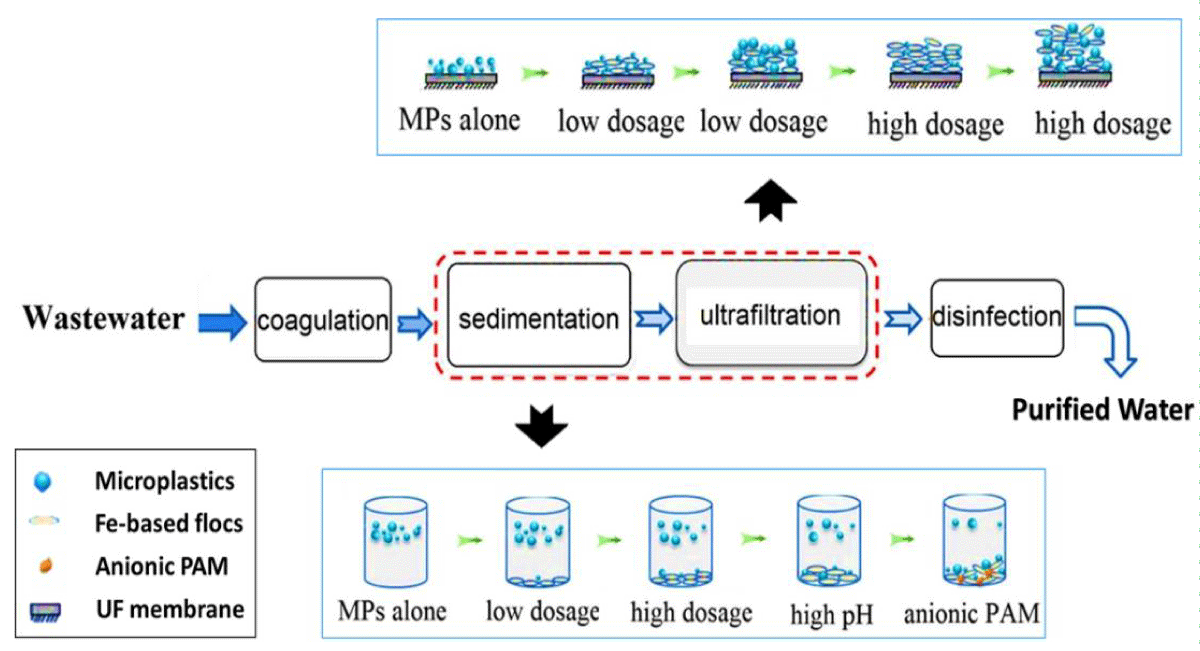
In addition, Ma, et al. (2019) used the same method to remove microplastics but applied a FeCl3.6H2O coagulation agent instead (Figure 5). Their experiments demonstrated that, at neutral pH, the removal of microplastics was enhanced with increasing concentrations of coagulants. This trend was especially clear for microplastics of less than 0.5 mm in diameter [48].
Figure 5: A coagulation, sedimentation, and ultrafiltration procedure for eliminating polyethylene microplastics from wastewater (modified from [48]).
In addition, the removal efficiency was further intensified with high pH and 2 molar mass (MM) coagulant concentrations, as well as for smaller microplastic particles. Under these conditions, anionic polyacrylamide performed far better than cationic polyacrylamide under a low dosage, 2 mM. As a result, the rates of removing microplastics from polyethylene were improved substantially. A mechanistic explanation can be put forward regarding the facile formation of Fe-based flocs during the coagulation process by using anionic polyacrylamides to make the products dense enough to be concentrated and trapped [48,71].
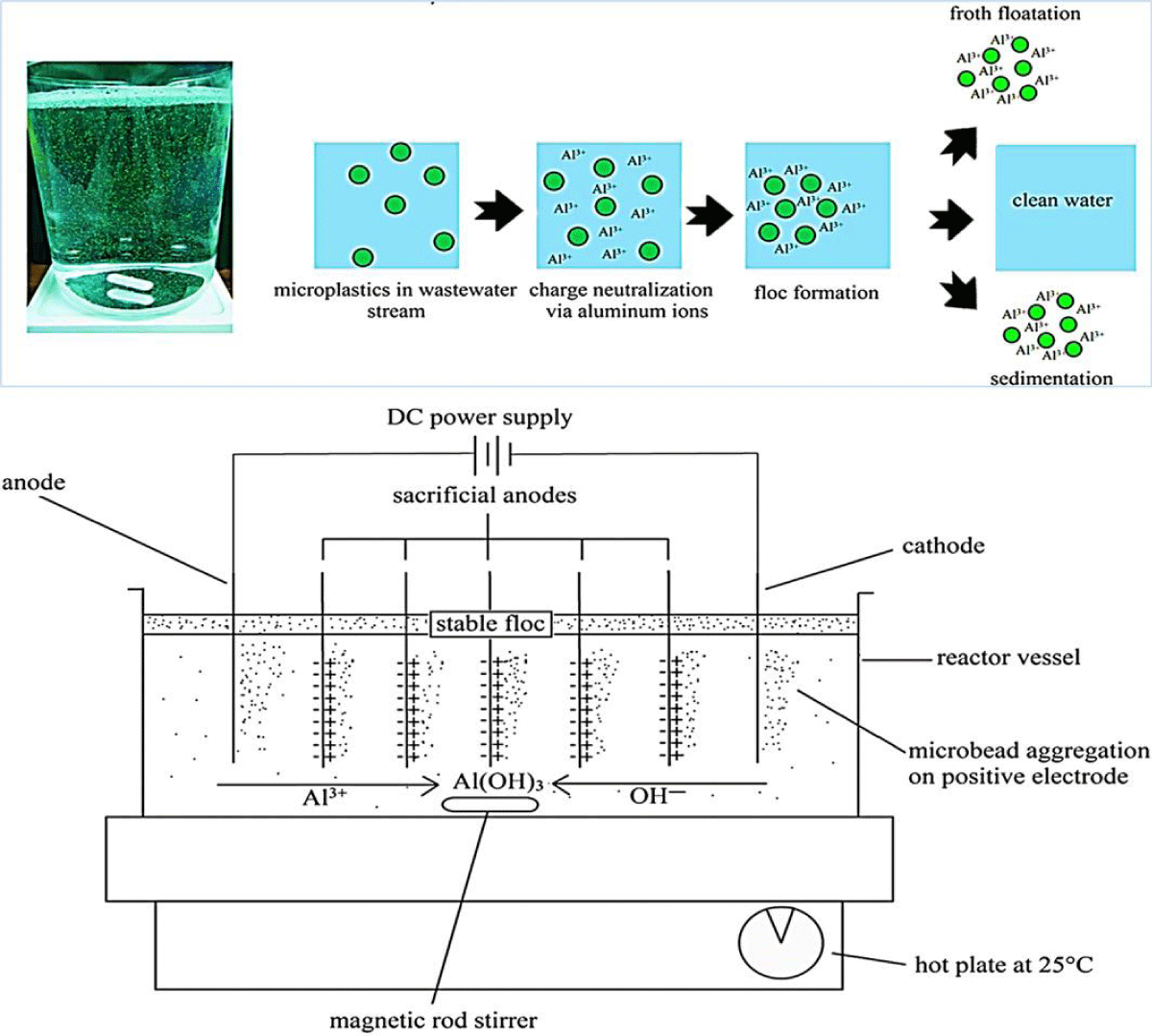
Several techniques have been successfully used by researchers to remove polyethylene microplastics from a stirred-tank batch reactor (Figure 6), including electrocoagulation, charge neutralization, and floc formation then to clean water with sedimentation, which is an environmentally safe, energy-efficient, cost-effective, and highly automated method [72].
Figure 6: An electrocoagulation reactor setup, in which Al3+ acts as a coagulation agent, is used for microplastic removal (modified from [72]).
In coagulation, colloids are broken apart and the surface charges of microparticles are stabilized. Through van der Waals forces, the particles can interact sufficiently close to one another [73]. Concurrently, the microplastics in the wastewater sample are trapped by the coagulants, forming a sludge blanket. Based on the results of all experiments using electrocoagulation, the removal efficiency was higher than 90%. Using pH 7.5 and NaCl concentrations between 0 and 2 g/L, 99.24% of the contaminants were removed. A further study found that the case of the 11 A/m2 tested current density, the lowest tested current density in terms of energy use, resulted in the highest removal rate [73,74].
Microplastics are not fully understood in terms of their degradation mechanisms. Brandon, et al. (2016), studied the chemical changes in the structure of polypropylene, polyethylene, and other microplastics throughout 3 years of simulated realistic weather conditions [75]. According to FTIR analyses, some metabolites, such as carbonyl, hydroxyl, and carbon-oxygen bonds, exhibited slight nonlinear changes with time, indicating that microplastics take a long time to degrade [75].
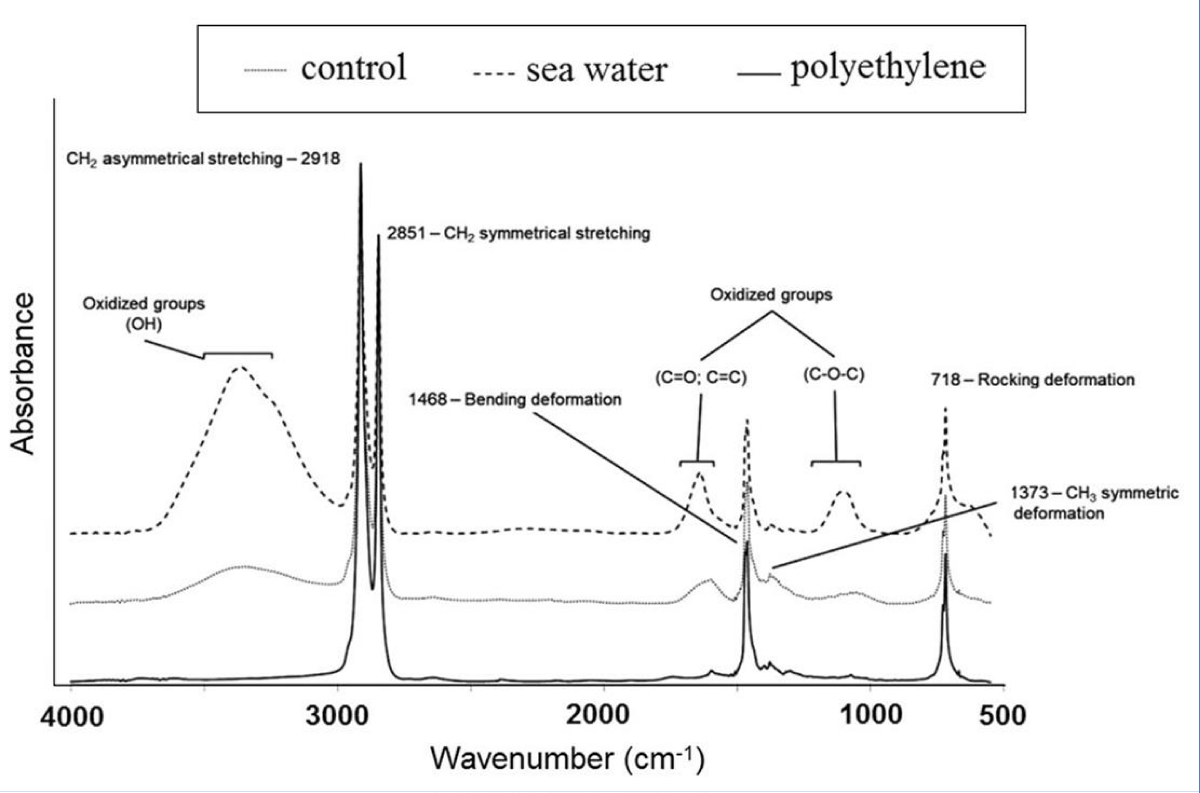
The degradation of microplastics by elements [76], microorganisms [11] and catalysts [77] has been extensively studied, but the degradation of microplastics has been relatively neglected. Liu, et al. (2019) investigated the long-term aging behaviors of polystyrene and polyethylene microplastics in the aquatic environment via a heat-activated persulfate-Fenton combined method [78]. They concluded that the O/C ratio and microplastic size were important factors determining microplastic adsorption capabilities and surface properties, which influence microplastic oxidation rates significantly [78]. Recently, studies have been published assessing the structural and morphological alterations of polyethylene microplastics under dark and UV light [11], [79]. According to FTIR analysis, artificial seawater was significantly more degradative than UV illumination compared to the initial materials and products (Figure 7).
Figure 7: Polyethylene microplastics FTIR spectra before and after being treated with artificial seawater for 8 weeks (modified from [79]).
A greater organic content in the medium confirmed this. Moreover, when microplastics were exposed to UV light for the same period, no critical changes in their chemical structure were observed, showing that salt is necessary to form oxidized sites. Microplastic surface morphology was also affected by salt, revealing observable cracking lines in SEM images. Such findings confirm the important role of salinity in microplastic degradation [1], [53,80-82].
According to Table 4, the following methods are used to remove microplastics from the environment: Here is a summary of the advantages and efficiency of recent projects. Microplastics should be removed from practical applications with membrane-based technologies.
| Table 4: Comparison of microplastic removal methods. | |||
| Methodology | The advantages | Efficiency% | Refs |
| Wastewater treatment plants (WWTP) |
Combination of sorption and biological processes that is simple to use, maintenance-free and highly efficient. | > 95 | [34] |
| Conventional activated sludge |
A flexible, robust, cost-effective system that will treat a wide range of influent concentrations, as well as large systems | 98 | [46] |
| Microalgae adsorption | Cut surfaces are highly affine to sorbing microplastic particles, and their surface charges are used to identify their selectivity. | 94.5 | [21] |
| Adaptable membranes | Simple operation, low trans-membrane pressure, and non-chemical treatment provide low filtration resistance. | > 90 | [54] |
| Aphotocatalytic degradation process | A renewable and pollution-free energy source that does not require additional chemicals, efficient mineralization, and environmental remediation that is environmentally friendly. | [83] | |
| Electrocoagulation | The system minimizes sludge accumulation, eliminates secondary pollution, and is energy efficient and cost-effective. It is also flexible to automate and has no chance of damaging the environment. | > 90 | [72] |
| Bioreactors with membranes |
Using porous membranes in conjunction with advanced treatment methods. | > 99 | [44] |
| Coagulation and agglomeration techniques | A simple mechanical device with controllable operational conditions for removing small microparticles. | 61 | [48,59] |
Detection, extraction, and removal of MPs pose challenges and gaps in the development of analytical methods [84]. As a result of the wide range of options available for MP sampling, detection, and analysis, it is currently very difficult to select the right method. This is especially true since it is a big challenge when dealing with wastewater samples [85]. There are a limited number of studies demonstrating that appropriate methodologies for identifying and removing MPs in WWTPs, wastewater, and sewage sludge are emerging [37]. Various removal techniques are used to remove MPs from wastewater effluents, but their exact fate, behavior, and mechanism of removal remain largely unknown [55]. In addition to having low MPs removal efficiency and a slow degradation rate, these technologies could generate unpredictable consequences, including toxicity, changes in topography, chemical characteristics, and secondary pollution [86]. There are several environmentally friendly, low-cost, and efficient methods for the removal of MP waste that have recently been reported [86], including solar energy, 3D solar evaporators, and photocatalysis [87].
Prospects for capturing MPs in the future: Based on the literature and numerous studies, it is clear that more details are needed, especially on practical figures such as reliable estimates of the fate, quantity, and human exposure to MPs [88]. A lack of solid definitions of MPs makes it difficult to compare the results of different surveys. The lack of a standard methodology for detection and identification is another challenge to obtaining comparable results. This method enables comparable results to those generated by these methods, according to Klein, et al. [82]. A major obstacle to large-scale monitoring is the time required for each phase of MP analysis, such as sampling, extraction, separation, and identification. This applies especially to MPs, for whom identification protocols are still lacking. As a result of effective and appropriate identification methodologies, the abundance and distribution of MPs will be more accurately assessed in the environment and aquatic environments, improving comparability between studies [89]. It may be necessary to adjust MP concentrations in exposure studies to ecologically realistic concentrations to avoid misinterpretation of unrealistic environmental results [89].
The mechanism by which MPs are removed from wastewater treatment processes has not been investigated yet. Advanced technologies have, however, been applied to these processes to improve removal efficiency. There is no specific information available about its role in removing MPs from treatment facilities that use membrane technology. Several questions arise regarding the economic viability of its use due to the high costs associated with its use. A better understanding of sustainable removal techniques is necessary, and more options for effective disposal in sewage processes that are considered inexpensive should be explored.
Despite the lack of clear information regarding MPs’ impact on the environment, it is crucial and urgent to consider, monitor, and avoid further pollution by MPs in wastewater [90]. The level and intensity of MP exposure are essential for assessing the impact on human health. A limited number of studies have been carried out on the environment, which leaves the field open to innovations [84]. It is a challenge to accumulate MPs in aquatic environments since they affect the habitat and disrupt the food web [91]. Future monitoring of material inventories may be made easier if MPs’ origins, routes, and pathways are identified and removed in soil, sediment, and water, or if wastewater treatment plants use more efficient equipment and environmentally friendly technologies [92].
Despite current efforts, plastic pollution continues to negatively impact the environment. Despite cleanup strategies that attempt to mitigate its effects, they cannot keep up with the growing amount of plastic entering the environment. The review recommends regulating plastic production and consumption, increasing recycled plastic demand, improving removal technologies, identifying sustainable removal technologies, converting MPs to renewable energy, educating consumers, and improving the lifecycle [93]. As a result of these recommendations, a circular economy can be implemented, and poorly managed pollutants will be reduced in the aquatic system [94].
In this review, major physiochemical approaches to removing microplastics have been summarized, a variety of methods are available to remove them from an environment, including chemical and physical methods. Microplastics are greatly reduced in influent water entering the treatment plants by a combination of filtration and membrane bioreactors, but these systems act as sources of microplastics every day because effluents are directly released into aquatic environments. The conventional activated sludge treatment strategy is used in water treatment plants as well as membrane bioreactor technologies, but it shows less efficiency than the latter method, which results in it being a less popular treatment method. Microplastics can also be effectively separated by electrocoagulation and agglomeration, but these techniques must be combined with significant additional filtration steps. To understand any structural alterations during degradation, FTIR and electron microscopy analysis are widely used. Based on the FTIR bands of the treated microplastics, it appears that the biological removal occurred via the oxidation of hydroperoxide and hydroxyl groups, carbonyl groups, and double bonds.
Ethical approval
The study was approved by Springer Ethics Committee (License Number 5244261023490) and approved by Prof. Eric Lichtfouse (Author) [email protected]
- Padervand M, Lichtfouse E, Robert D, Wang C. Removal of microplastics from the environment. A review. Environ Chem Lett. 2020; 18: 807-828. doi: 10.1007/s10311-020-00983-1.
- Al-Rawajfeh AE, AlShamaileh EM, Alrbaihat MR. Clean and efficient synthesis using mechanochemistry: Preparation of kaolinite–KH 2 PO 4 and kaolinite–(NH 4 ) 2 HPO 4 complexes as slow released fertilizer. J Ind Eng Chem. 2019; 73: 336–343. 2019.
- Kumar A, Yedhu Krishnan R. A Review on the Technology of Size Reduction Equipment. Int J ChemTech Res. 2020; 13: 48-54. doi: 10.20902/ijctr.2019.130106.
- Esnouf A, Latrille É, Steyer JP, Helias A. Representativeness of environmental impact assessment methods regarding Life Cycle Inventories. Sci Total Environ. 2018 Apr 15;621:1264-1271. doi: 10.1016/j.scitotenv.2017.10.102. Epub 2017 Oct 19. PMID: 29055597.
- Van Cauwenberghe L, Vanreusel A, Mees J, Janssen CR. Microplastic pollution in deep-sea sediments. Environ Pollut. 2013 Nov;182:495-9. doi: 10.1016/j.envpol.2013.08.013. Epub 2013 Sep 12. PMID: 24035457.
- Hirai H, Takada H, Ogata Y, Yamashita R, Mizukawa K, Saha M, Kwan C, Moore C, Gray H, Laursen D, Zettler ER, Farrington JW, Reddy CM, Peacock EE, Ward MW. Organic micropollutants in marine plastics debris from the open ocean and remote and urban beaches. Mar Pollut Bull. 2011 Aug;62(8):1683-92. doi: 10.1016/j.marpolbul.2011.06.004. Epub 2011 Jun 29. PMID: 21719036.
- Faure F, Demars C, Wieser O, Kunz M, de Alencastro LF. Plastic pollution in Swiss surface waters: nature and concentrations, interaction with pollutants. (Special Issue: Microplastics in the environment.). Environ Chem. 2015; 12: 582–591.
- Law KL, Morét-Ferguson SE, Goodwin DS, Zettler ER, Deforce E, Kukulka T, Proskurowski G. Distribution of surface plastic debris in the eastern Pacific Ocean from an 11-year data set. Environ Sci Technol. 2014 May 6;48(9):4732-8. doi: 10.1021/es4053076. Epub 2014 Apr 24. PMID: 24708264.
- Andrady AL. Microplastics in the marine environment. Mar Pollut Bull. 2011; 62: 1596-1605. doi: 10.1016/j.marpolbul.2011.05.030.
- Noyma NP, de Magalhães L, Furtado LL, Mucci M, van Oosterhout F, Huszar VL, Marinho MM, Lürling M. Controlling cyanobacterial blooms through effective flocculation and sedimentation with combined use of flocculants and phosphorus adsorbing natural soil and modified clay. Water Res. 2016 Jun 15;97:26-38. doi: 10.1016/j.watres.2015.11.057. Epub 2015 Dec 8. PMID: 26706124.
- Pathak VM, Navneet, Review on the current status of polymer degradation: a microbial approach. Bioresour Bioprocess. 2017; 4. doi: 10.1186/s40643-017-0145-9.
- Li J, Liu H, Paul Chen J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018 Jun 15;137:362-374. doi: 10.1016/j.watres.2017.12.056. Epub 2017 Dec 28. PMID: 29580559.
- Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet ME, Le Goïc N, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci U S A. 2016 Mar 1;113(9):2430-5. doi: 10.1073/pnas.1519019113. Epub 2016 Feb 1. PMID: 26831072; PMCID: PMC4780615.
- Meeker JD, Sathyanarayana S, Swan SH. Phthalates and other additives in plastics: human exposure and associated health outcomes. Philos Trans R Soc Lond B Biol Sci. 2009 Jul 27;364(1526):2097-113. doi: 10.1098/rstb.2008.0268. PMID: 19528058; PMCID: PMC2873014.
- Piringer OG, Baner AL. Permeation of gases, water vapor and volatile organic compounds. Plast Packag Mater food barrier Funct. mass Transp. Qual. Assur. Legis. 2000; 250.
- Lau OW, Wong SK. Contamination in food from packaging material. J Chromatogr A. 2000; 882: 255-270.
- Nobre CR, Santana MFM, Maluf A, Cortez FS, Cesar A, Pereira CDS, Turra A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar Pollut Bull. 2015 Mar 15;92(1-2):99-104. doi: 10.1016/j.marpolbul.2014.12.050. Epub 2015 Feb 7. PMID: 25662316.
- Wang W, Ndungu AW, Li Z, Wang J. Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of Wuhan, China. Sci Total Environ. 2017 Jan 1;575:1369-1374. doi: 10.1016/j.scitotenv.2016.09.213. Epub 2016 Sep 29. PMID: 27693147.
- Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann Intern Med. 2019 Oct 1;171(7):453-457. doi: 10.7326/M19-0618. Epub 2019 Sep 3. PMID: 31476765.
- Vickers NJ. Animal Communication: When I'm Calling You, Will You Answer Too? Curr Biol. 2017 Jul 24;27(14):R713-R715. doi: 10.1016/j.cub.2017.05.064. PMID: 28743020.
- Zhang C, Chen X, Wang J, Tan L. Toxic effects of microplastic on marine microalgae Skeletonema costatum: Interactions between microplastic and algae. Environ Pollut. 2017 Jan;220(Pt B):1282-1288. doi: 10.1016/j.envpol.2016.11.005. Epub 2016 Nov 18. PMID: 27876228.
- Au SY, Bruce TF, Bridges WC, Klaine SJ. Responses of Hyalella azteca to acute and chronic microplastic exposures. Environ Toxicol Chem. 2015 Nov;34(11):2564-72. doi: 10.1002/etc.3093. Epub 2015 Sep 23. PMID: 26042578.
- Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res. 2016 Sep;120:1-8. doi: 10.1016/j.marenvres.2016.07.004. Epub 2016 Jul 5. PMID: 27411093.
- Prata JC. Airborne microplastics: Consequences to human health? Environ Pollut. 2018 Mar;234:115-126. doi: 10.1016/j.envpol.2017.11.043. Epub 2017 Dec 21. PMID: 29172041.
- Jiang JQ. Occurrence of microplastics and its pollution in the environment: A review. Sustain Prod Consum. 2018; 13: 16–23. doi: 10.1016/j.spc.2017.11.003.
- Besseling E, Wang B, Lürling M, Koelmans AA. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environ Sci Technol. 2014 Oct 21;48(20):12336-43. doi: 10.1021/es503001d. Epub 2014 Oct 10. Erratum in: Environ Sci Technol. 2014 Dec 2;48(23):14065. PMID: 25268330; PMCID: PMC6863593.
- Fasano E, Bono-Blay F, Cirillo T, Montuori P, Lacorte S. Migration of phthalates, alkylphenols, bisphenol A and di(2-ethylhexyl)adipate from food packaging. Food Control. 2012; 27: 132–138.
- Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philos Trans R Soc Lond B Biol Sci. 2009 Jul 27;364(1526):2079-96. doi: 10.1098/rstb.2008.0281. PMID: 19528057; PMCID: PMC2873015.
- Wright SL, Kelly FJ. Plastic and Human Health: A Micro Issue? Environ Sci Technol. 2017 Jun 20;51(12):6634-6647. doi: 10.1021/acs.est.7b00423. Epub 2017 Jun 7. PMID: 28531345.
- Hamidian AH, Ozumchelouei EJ, Feizi F, Wu C, Zhang Y, Yang M. A review on the characteristics of microplastics in wastewater treatment plants: a source for toxic chemicals. J Clean Prod. 2021; 295: 126480.
- Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J. Plastic Pollution in the World's Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS One. 2014 Dec 10;9(12):e111913. doi: 10.1371/journal.pone.0111913. PMID: 25494041; PMCID: PMC4262196.
- Thompson RC, Moore CJ, vom Saal FS, Swan SH. Plastics, the environment and human health: current consensus and future trends. Philos Trans R Soc Lond B Biol Sci. 2009 Jul 27;364(1526):2153-66. doi: 10.1098/rstb.2009.0053. PMID: 19528062; PMCID: PMC2873021.
- Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R. Accumulation of microplastic on shorelines woldwide: sources and sinks. Environ Sci Technol. 2011 Nov 1;45(21):9175-9. doi: 10.1021/es201811s. Epub 2011 Oct 4. PMID: 21894925.
- Long Z, Pan Z, Wang W, Ren J, Yu X, Lin L, Lin H, Chen H, Jin X. Microplastic abundance, characteristics, and removal in wastewater treatment plants in a coastal city of China. Water Res. 2019 May 15;155:255-265. doi: 10.1016/j.watres.2019.02.028. Epub 2019 Feb 25. PMID: 30852313.
- Murphy F, Ewins C, Carbonnier F, Quinn B. Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment. Environ Sci Technol. 2016 Jun 7;50(11):5800-8. doi: 10.1021/acs.est.5b05416. Epub 2016 May 18. PMID: 27191224.
- Jin L, Zhang G, Tian H. Current state of sewage treatment in China. Water Res. 2014 Dec 1;66:85-98. doi: 10.1016/j.watres.2014.08.014. Epub 2014 Aug 22. PMID: 25189479.
- Sun J, Dai X, Wang Q, van Loosdrecht MCM, Ni BJ. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019 Apr 1;152:21-37. doi: 10.1016/j.watres.2018.12.050. Epub 2019 Jan 2. PMID: 30660095.
- Carr SA, Liu J, Tesoro AG. Transport and fate of microplastic particles in wastewater treatment plants. Water Res. 2016 Mar 15;91:174-82. doi: 10.1016/j.watres.2016.01.002. Epub 2016 Jan 7. PMID: 26795302.
- Bui XT, Nguyen PT, Nguyen VT, Dao TS, Nguyen PD. Microplastics pollution in wastewater: Characteristics, occurrence and removal technologies. Environ Technol Innov. 2020; 19: 101013.
- Magni S, Binelli A, Pittura L, Avio CG, Della Torre C, Parenti CC, Gorbi S, Regoli F. The fate of microplastics in an Italian Wastewater Treatment Plant. Sci Total Environ. 2019 Feb 20;652:602-610. doi: 10.1016/j.scitotenv.2018.10.269. Epub 2018 Oct 21. PMID: 30368189.
- Hidayaturrahman H, Lee TG. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar Pollut Bull. 2019 Sep;146:696-702. doi: 10.1016/j.marpolbul.2019.06.071. Epub 2019 Jul 19. PMID: 31426211.
- Östman M, Björlenius B, Fick J, Tysklind M. Effect of full-scale ozonation and pilot-scale granular activated carbon on the removal of biocides, antimycotics and antibiotics in a sewage treatment plant. Sci Total Environ. 2019 Feb 1;649:1117-1123. doi: 10.1016/j.scitotenv.2018.08.382. Epub 2018 Aug 28. PMID: 30308883.
- Talvitie J, Mikola A, Koistinen A, Setälä O. Solutions to microplastic pollution - Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017 Oct 15;123:401-407. doi: 10.1016/j.watres.2017.07.005. Epub 2017 Jul 2. PMID: 28686942.
- Lares M, Ncibi MC, Sillanpää M, Sillanpää M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018 Apr 15;133:236-246. doi: 10.1016/j.watres.2018.01.049. Epub 2018 Feb 3. PMID: 29407704.
- Anastasiou E, Lorentz KO, Stein GJ, Mitchell PD. Prehistoric schistosomiasis parasite found in the Middle East. Lancet Infect Dis. 2014 Jul;14(7):553-4. doi: 10.1016/S1473-3099(14)70794-7. PMID: 24953264.
- Gurung K, Ncibi MC, Fontmorin JM. Incorporating Submerged MBR in Conventional Activated Sludge Process for Municipal Wastewater Treatment: A Feasibility and Performance Assessment. J Membr Sci Technol. 2016; 6. doi: 10.4172/2155-9589.1000158.
- Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017 Jan 1;108:365-372. doi: 10.1016/j.watres.2016.11.015. Epub 2016 Nov 4. PMID: 27838027.
- Ma B, Xue W, Ding Y, Hu C, Liu H, Qu J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J Environ Sci (China). 2019 Apr;78:267-275. doi: 10.1016/j.jes.2018.10.006. Epub 2018 Oct 30. PMID: 30665645.
- Horton AA, Dixon SJ. Microplastics: An introduction to environmental transport processes. WIREs Water. 2018; 5: 1-10.
- Yang L, Li K, Cui S, Kang Y, An L, Lei K. Removal of microplastics in municipal sewage from China's largest water reclamation plant. Water Res. 2019 May 15;155:175-181. doi: 10.1016/j.watres.2019.02.046. Epub 2019 Feb 26. PMID: 30849731.
- Talvitie J, Mikola A, Setälä O, Heinonen M, Koistinen A. How well is microlitter purified from wastewater? - A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant. Water Res. 2017 Feb 1;109:164-172. doi: 10.1016/j.watres.2016.11.046. Epub 2016 Nov 16. PMID: 27883921.
- Ersahin ME, Tao Y, Ozgun H, Gimenez JB, Spanjers H, van Lier JB. Impact of anaerobic dynamic membrane bioreactor configuration on treatment and filterability performance. J Memb Sci. 2016; 526: 387-394. doi: 10.1016/j.memsci.2016.12.057.
- Andrady AL. The plastic in microplastics: A review. Mar Pollut Bull. 2017 Jun 15;119(1):12-22. doi: 10.1016/j.marpolbul.2017.01.082. Epub 2017 Apr 24. PMID: 28449819.
- Li L, Xu G, Yu H, Xing J. Dynamic membrane for micro-particle removal in wastewater treatment: Performance and influencing factors. Sci Total Environ. 2018 Jun 15;627:332-340. doi: 10.1016/j.scitotenv.2018.01.239. Epub 2018 Feb 3. PMID: 29426156.
- Talvitie J, Mikola A, Koistinen A, Setälä O. Solutions to microplastic pollution - Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017 Oct 15;123:401-407. doi: 10.1016/j.watres.2017.07.005. Epub 2017 Jul 2. PMID: 28686942.
- Raza W, Lee J, Raza N, Luo Y, Kim KH, Yang J. Removal of phenolic compounds from industrial waste water based on membrane-based technologies. J Ind Eng Chem. 2019; 71: 1-18.
- Cole M, Lindeque P, Halsband C, Galloway TS. Microplastics as contaminants in the marine environment: a review. Mar Pollut Bull. 2011 Dec;62(12):2588-97. doi: 10.1016/j.marpolbul.2011.09.025. Epub 2011 Oct 14. PMID: 22001295.
- Graham ER, Thompson JT. Deposit- and suspension-feeding sea cucumbers (Echinodermata) ingest plastic fragments. J Exp Mar Bio Ecol. 2009; 368: 22-29.
- Wang F. Effects of nanoparticles on algae: Adsorption, distribution, ecotoxicity and fate. Appl Sci. 2019; 9: 1534.
- Rios LM, Moore C, Jones PR. Persistent organic pollutants carried by synthetic polymers in the ocean environment. Mar Pollut Bull. 2007 Aug;54(8):1230-7. doi: 10.1016/j.marpolbul.2007.03.022. Epub 2007 May 29. PMID: 17532349.
- Baur X. Health risks in international container and bulk cargo transport due to volatile toxic compounds. J Occup Med Toxicol. 2015; 10: 1-18.
- Sundbæk KB, Koch IDW, Villaro CG, Rasmussen NS, Holdt SL, Hartmann NB. Sorption of fluorescent polystyrene microplastic particles to edible seaweed Fucus vesiculosus. J Appl Phycol. 2018; 30: 2923–2927. doi: 10.1007/s10811-018-1472-8.
- Martins MJ, Mota CF, Pearson GA. Sex-biased gene expression in the brown alga Fucus vesiculosus. BMC Genomics. 2013 May 1;14:294. doi: 10.1186/1471-2164-14-294. PMID: 23634783; PMCID: PMC3652789.
- Bhattacharya P, Lin S, Turner JP, Ke PC. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis. J Phys Chem C. 2010; 114: 16556-16561.
- Nolte TM, Hartmann NB, Kleijn JM, Garnæs J, van de Meent D, Jan Hendriks A, Baun A. The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption. Aquat Toxicol. 2017 Feb;183:11-20. doi: 10.1016/j.aquatox.2016.12.005. Epub 2016 Dec 8. PMID: 27978483.
- Cheng SY, Show PL, Lau BF, Chang JS, Ling TC. New Prospects for Modified Algae in Heavy Metal Adsorption. Trends Biotechnol. 2019 Nov;37(11):1255-1268. doi: 10.1016/j.tibtech.2019.04.007. Epub 2019 Jun 4. PMID: 31174882.
- Lee KE, Morad N, Teng TT, Poh BT. Development, characterization and the application of hybrid materials in coagulation/flocculation of wastewater: A review. Chem Eng J. 2012; 203: 370-386.
- Chorghe D, Sari MA, Chellam S. Boron removal from hydraulic fracturing wastewater by aluminum and iron coagulation: Mechanisms and limitations. Water Res. 2017 Dec 1;126:481-487. doi: 10.1016/j.watres.2017.09.057. Epub 2017 Sep 28. PMID: 29028491.
- Ariza-Tarazona MC, Villarreal-Chiu JF, Barbieri V, Siligardi C, Cedillo-González EI. New strategy for microplastic degradation: Green photocatalysis using a protein-based porous N-TiO 2 semiconductor. Ceram Int. 2019; 45: 9618-9624. doi: 10.1016/j.ceramint.2018.10.208.
- Liu W, Zhang J, Liu H, Guo X, Zhang X, Yao X, Cao Z, Zhang T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ Int. 2021 Jan;146:106277. doi: 10.1016/j.envint.2020.106277. Epub 2020 Nov 20. PMID: 33227584.
- Sarkar DJ, Das Sarkar S, Das BK, Praharaj JK, Mahajan DK, Purokait B, Mohanty TR, Mohanty D, Gogoi P, Kumar V S, Behera BK, Manna RK, Samanta S. Microplastics removal efficiency of drinking water treatment plant with pulse clarifier. J Hazard Mater. 2021 Jul 5;413:125347. doi: 10.1016/j.jhazmat.2021.125347. Epub 2021 Feb 9. PMID: 33601144.
- Perren W, Wojtasik A, Cai Q. Removal of Microbeads from Wastewater Using Electrocoagulation. ACS Omega. 2018 Mar 20;3(3):3357-3364. doi: 10.1021/acsomega.7b02037. PMID: 31458591; PMCID: PMC6641227.
- Akbal F, Camcidotless S. Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination; 2011; 269: 214–222.
- Zhou G, Wang Q, Li J, Li Q, Xu H, Ye Q, Wang Y, Shu S, Zhang J. Removal of polystyrene and polyethylene microplastics using PAC and FeCl3 coagulation: Performance and mechanism. Sci Total Environ. 2021 Jan 15;752:141837. doi: 10.1016/j.scitotenv.2020.141837. Epub 2020 Aug 20. PMID: 32889273.
- Brandon J, Goldstein M, Ohman MD. Long-term aging and degradation of microplastic particles: Comparing in situ oceanic and experimental weathering patterns. Mar Pollut Bull. 2016 Sep 15;110(1):299-308. doi: 10.1016/j.marpolbul.2016.06.048. Epub 2016 Jun 22. PMID: 27344287.
- Colom X, Cañavate J, Suñol JJ, Pagès P, Saurina J, Carrasco F. Natural and artificial aging of polypropylene-polyethylene copolymers. J Appl Polym Sci. 2002; 87: 1685–1692, 2002, doi: 10.1002/app.11613.
- Hazrat MA, Rasul MG, Khan MMK. A study on thermo-catalytic degradation for production of clean transport fuel and reducing plastic wastes. Procedia Eng. 2014; 105:. 865–876.
- Liu P, Qian L, Wang H, Zhan X, Lu K, Gu C, Gao S. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ Sci Technol. 2019 Apr 2;53(7):3579-3588. doi: 10.1021/acs.est.9b00493. Epub 2019 Mar 13. PMID: 30829479.
- Da Costa JP, Nunes AR, Santos PSM, Girão AV, Duarte AC, Rocha-Santos T. Degradation of polyethylene microplastics in seawater: Insights into the environmental degradation of polymers. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2018 Jul 29;53(9):866-875. doi: 10.1080/10934529.2018.1455381. Epub 2018 Apr 6. PMID: 29624466.
- Zhang K, Hamidian AH, Tubić A, Zhang Y, Fang JKH, Wu C, Lam PKS. Understanding plastic degradation and microplastic formation in the environment: A review. Environ Pollut. 2021 Apr 1;274:116554. doi: 10.1016/j.envpol.2021.116554. Epub 2021 Jan 23. PMID: 33529891.
- Du H, Xie Y, Wang J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J Hazard Mater. 2021 Sep 15;418:126377. doi: 10.1016/j.jhazmat.2021.126377. Epub 2021 Jun 10. PMID: 34130168.
- Klein S, Dimzon IK, Eubeler J, Knepper TP. Analysis, occurrence, and degradation of microplastics in the aqueous environment,” in Freshwater microplastics, Springer, Cham 2018. 51–67.
- Tofa TS, Kunjali KL, Paul S, Dutta J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ Chem Lett. 2019; 17: 1341-1346.
- Rostami S, Talaie MR, Talaiekhozani A, Sillanpää M. Evaluation of the available strategies to control the emission of microplastics into the aquatic environment. Environ Sci Pollut Res Int. 2021 Apr;28(15):18908-18917. doi: 10.1007/s11356-021-12888-9. Epub 2021 Feb 17. PMID: 33594573.
- Prata JC, Reis V, Matos JTV, da Costa JP, Duarte AC, Rocha-Santos T. A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). Sci Total Environ. 2019 Nov 10;690:1277-1283. doi: 10.1016/j.scitotenv.2019.07.060. Epub 2019 Jul 5. PMID: 31470490.
- Wang P. Sustainable removal of nano/microplastics in water by solar energy. Chem Eng J. 2022; 428: 131196.
- Canopoli L, Coulon F, Wagland ST. Degradation of excavated polyethylene and polypropylene waste from landfill. Sci Total Environ. 2020 Jan 1;698:134125. doi: 10.1016/j.scitotenv.2019.134125. Epub 2019 Aug 26. PMID: 31783451.
- Alexy P, Anklam E, Emans T, Furfari A, Galgani F, Hanke G, Koelmans A, Pant R, Saveyn H, Sokull Kluettgen B. Managing the analytical challenges related to micro- and nanoplastics in the environment and food: filling the knowledge gaps. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2020 Jan;37(1):1-10. doi: 10.1080/19440049.2019.1673905. Epub 2019 Oct 9. PMID: 31596687.
- Yu P, Liu Z, Wu D, Chen M, Lv W, Zhao Y. Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat Toxicol. 2018 Jul;200:28-36. doi: 10.1016/j.aquatox.2018.04.015. Epub 2018 Apr 24. PMID: 29709883.
- Ballent A, Corcoran PL, Madden O, Helm PA, Longstaffe FJ. Sources and sinks of microplastics in Canadian Lake Ontario nearshore, tributary and beach sediments. Mar Pollut Bull. 2016 Sep 15;110(1):383-395. doi: 10.1016/j.marpolbul.2016.06.037. Epub 2016 Jun 21. PMID: 27342902.
- Setälä O, Fleming-Lehtinen V, Lehtiniemi M. Ingestion and transfer of microplastics in the planktonic food web. Environ Pollut. 2014 Feb;185:77-83. doi: 10.1016/j.envpol.2013.10.013. Epub 2013 Nov 9. PMID: 24220023.
- Tang KHD, Hadibarata T. Microplastics removal through water treatment plants: Its feasibility, efficiency, future prospects and enhancement by proper waste management. Environ Challenges. 2021; 5: 100264.
- Hartley BL, Pahl S, Holland M, Alampei I, Veiga JM, Thompson RC. Turning the tide on trash: Empowering European educators and school students to tackle marine litter. Mar Policy. 2018; 96: 227-234.
- Sadia M. Microplastics pollution from wastewater treatment plants: A critical review on challenges, detection, sustainable removal techniques and circular economy. Environ Technol Innov. 2022; 102946.