More Information
Submitted: January 30, 2023 | Approved: March 13, 2023 | Published: March 14, 2023
How to cite this article: Imanova G, Bekpulatov I, Aliyev A, Barkaoui S. Importance of the radiations in water splitting for hydrogen generation. Ann Adv Chem. 2023; 7: 031-036.
DOI: 10.29328/journal.aac.1001040
Copyright License: © 2023 Imanova G, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Radiolysis; Hydrogen generation; Radiations; Future perspectives; Safety measures
Importance of the radiations in water splitting for hydrogen generation
Gunel Imanova1*, Ilkhom Bekpulatov2, Anar Aliyev1 and Sami Barkaoui3
1Institute of Radiation Problems, Ministry of Science and Education Republic of Azerbaijan, Baku AZ-1143, Azerbaijan
2Tashkent State Technical University, Tashkent, Uzbekistan
3Materials Processing and Analysis Laboratory INRAP, Technopôle, Sidi Thabet 2020, Tunisia
*Address for Correspondence: Gunel Imanova, Institute of Radiation Problems, Ministry of Science and Education Republic of Azerbaijan, Baku AZ-1143, Azerbaijan, Email: [email protected]
The review article examines the production of molecular hydrogen from the decomposition of water by various irradiation methods. The article shows different types of radiation: UV radiation, visible radiation, gamma radiation, X-ray radiation and neutron radiation. Electrons generated by radiation inside a nanoparticle of radius R suspense in fluid water are diffused with equal probability in all directions inside the particle and gradually lose their kinetic energy as a result of elastic and inelastic collisions. Some of these electrons are transported to the nanoparticle surface during the physical and physicochemical stages of the process and emitted into the water. It is extremely important for the formation of nanostructured materials after exposure to ordered nanostructure from the new phase with a period of a few nanometers, promoting the preservation of the properties of materials under high irradiation.
To ensure safety, it is important to be sure of the tightness of the system during the use of hydrogen in terms of preventing accidents and studying these processes by developing methods of accident management can diminish the adverse effects. The literature review shows that the theoretical and experimental results of these processes are an excellent basis for process management, safe operation of reactors and safety of hydrogen energy.
This should be taken into account in the study and analysis of oxidation processes under the influence of irradiation. All the considerations about the effect of radiolysis on oxidation processes are justified by the effect on the kinetics of oxidation processes in the aquatic environment [1,2].
Thus, in water and nuclear reactors, in the fields of radiation materials science, the composition of construction materials, storage containers and the lifespan of nuclear waste is in contact with solid surfaces of waste materials and the study of hydrogen explosives in H2O2 leads to problem-solving as a result [3,4].
Based on these results, the increase in the formation of molecular hydrogen occurs in the presence of oxide and water systems. Oxygen production requires less energy than hydrogen. The radiolysis process of GeO2 generates helium ion radicals at 5 MeV, which is lower than ZrO2 in the formation of molecular hydrogen as a result of γ-rays [5].
The resulting radicals weaken their oxidation and cause their destruction. Otherwise, the total potential of hydroxyl radicals (OH·) has very strong oxidizing properties and is equal to E° (HO*/ H2O) = + 2.7 eV. Various radicals are formed as a result of radiolysis processes. However, these reactions are time-depended and have been obtained experimentally. The mechanism explaining the received results is offered [6].
The kinetics of molecular hydrogen accumulation at gamma radiolysis of water on the n-ZrO2 surface is investigated. The influence of gamma radiations on n-ZrO2+water systems is studied at various temperatures T = 300…673 K [7].
In the temperature range 300 ≤ T ≤ 473 K, the activation energy of the radiation-thermal process is 1.07 kJ/mol, and in the temperature range 573 ≤ T ≤ 673 K, the activation energy of the thermal and radiation-thermal processes is 68.6 and 53.83 kJ/mol, respectively [8,9].
The amount, formation rate, and radiation-chemical yield of molecular hydrogen obtained from the water radiolysis process within the system have been defined according to both water and BeO by maintaining the water volume constant (V = 5 ml); by changing the mass (mBeO = 0.0 (pure water), 0.01; 0.02; 0.04; 0.08; 0.2 g) and particle size (d < 4, d = 32…53 and 75…106 μm) of beryllium oxide in the porous BeO/H2O suspended systems by the influence of γ-quantum [10].
Photochemistry of water splitting
Herein, the primary systems prevent the spread of oxides as a result of the oxidation of hydrogen and remain relevant for use in high-pressure water-cooled reactors. The reunification of H2 and H2O2 into water indicates the combination of H+ ions and OH* radicals. Under the influence of radiation, the chain reaction forms H* and OH* radicals at very low concentrations. Therefore, the breakdown of molecular hydrogen leads to the production of these compounds [11,12].
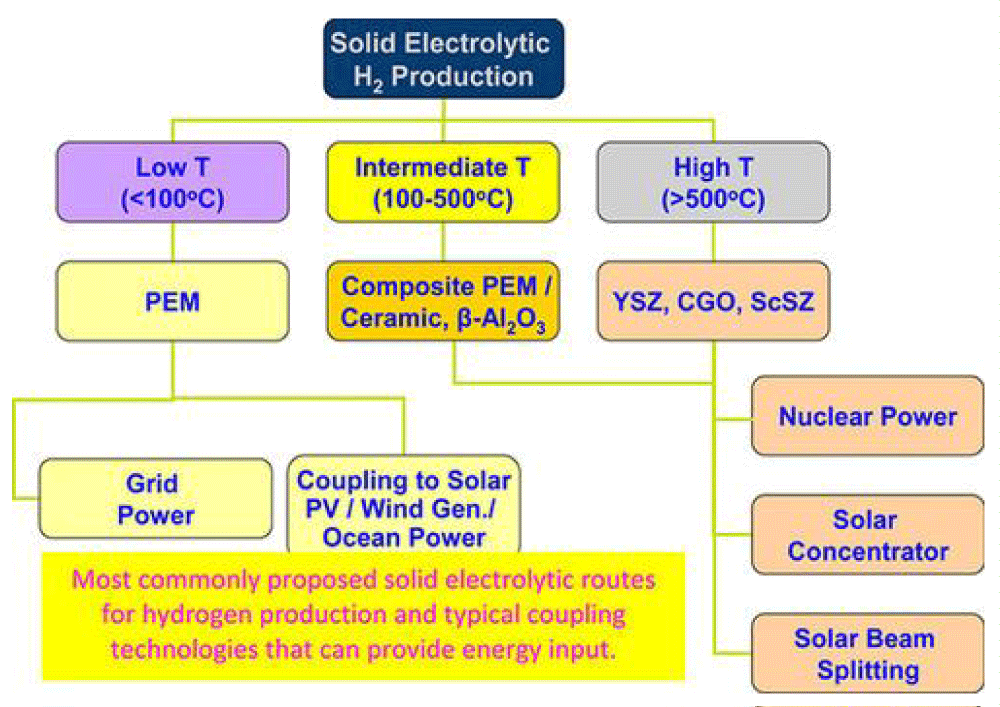
In heterogeneous systems, the process of water decomposition takes place in three stages. In these cases, the ways of energy reduction and energy transfer are subjected to ionizing radiation and physical and physicochemical reversible reactions. Thus, changes in the surface of nanoparticle oxide samples indicate the relevance of nanoparticles and are important for these physicochemical processes (Figure 1).
Figure 1: Most commonly proposed solid electrolytic routes for hydrogen production and typical coupling technologies that can provide energy input [13].
Energy transfer occurs during the adsorption process under the influence of high-energy radiation on the surface of nanoparticle oxide samples. These reactions can be considered “radiation catalysis”. The production of molecular hydrogen in the process of radiolysis in the oxide-water system has been shown in numerous studies [13,14].
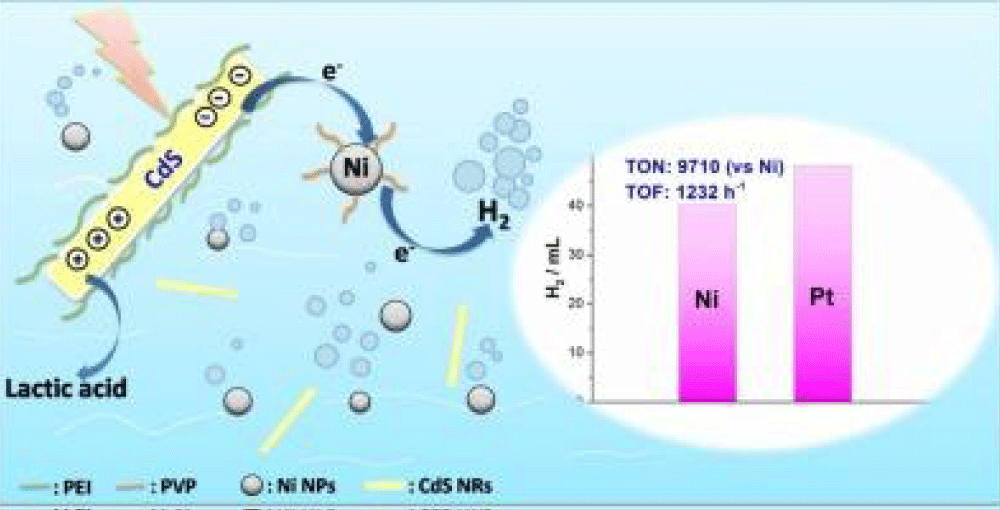
One of the main issues in conducting such research is the creation of new fuels and construction materials, and the discovery of new methods of material analysis control (Figure 2).
Figure 2: A possible mechanism for photocatalytic hydrogen evolution [15].
Nanostructures refer to macroscopic materials that contain nanoscale elements. The initial formation of atoms with dimensions of 0.1 ÷ 1 nm (10 ÷ 104) is observed in nanoclusters. Nanoparticles are usually used for small molecular nanoclusters with characteristic dimensions of atoms (molecules) to form intermediate products < 100 nm [15-17].
If the properties of the clusters do not depend on the number of atoms, it can be assumed that a small amount of macroscopic material has been obtained. Examples of nanosystems smaller than 100 nm are clusters with 10 to 104 atoms. In two-dimensional objects, for example, nanostructures smaller than 100 nm, such as graphene and carbon nanotubes [18].
About 1% of the STE particles obtained are the values of the electron-hole centers. Many activities occur as a result of different STE groups and multiple interactions. The centers of the holes exposed to gamma radiation as a result of the processes have been studied by many scientists. The formation of an oxygen molecule as a result of the irradiation of metal samples is shown in this study. It is possible to show some of the mechanisms of obtaining a metal oxygen molecule as a result of radiation, which also exists as a result of reactions and is written in the form (“Me-O-O-Me”) [19].
The monotony of the process must be broken in order to establish the kinetic parameters. The likelihood of encountering such cases usually occurs in rapid reactions. However, in this case, the situation becomes more complicated, when catalysts and inhibitors are used [20,21].
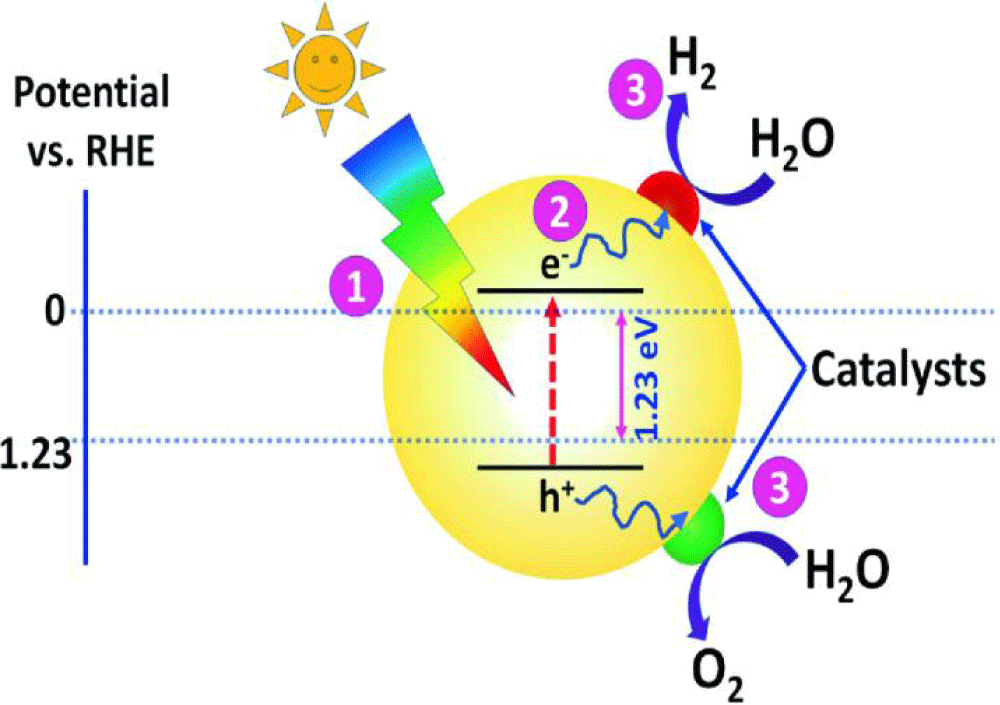
In oxide systems, it is almost more appropriate to obtain molecular hydrogen. Therefore, it is important to accelerate the decomposition reactions with free and pure water HO*. In these systems, diffusion occurs in limited numbers between H2 and HO*. As a result of the positive effect of catalysts, diffusion coefficients are reduced. The photochemical water splitting to produce O2 and H2 is considered the most promising, sustainable, renewable, and cost-effective energy technology for the future. In the photochemical water splitting process, the efficiency of H2 and O2 production rates depend on the properties of the selected semiconductor material. However, most semiconductors face various limitations which confine their water-splitting efficiency. Different strategies could be implemented to improve the water-splitting efficiency of semiconductors (Figure 3).
Figure 3: Electro-catalysts for Photochemical Water-Splitting [19-22].
Among them, loading of catalyst onto the water-splitting material is known to be one of the effective strategies to enhance the H2 and O2 production rates. Given this, several catalytic materials have been explored and successfully utilized in efficient O2 and H2 production systems. The surface deposited catalysts were known to reduce the surface trap states, which decreases the charge recombination and acts as a protective layer to minimize photo-corrosion of the light-absorbing semiconductors. Conclusively, exploring the efficient catalysts for photochemical water splitting requires more research contribution toward the understanding of the core reaction mechanism of the catalytic process with the use of sustainable and stable materials.
Hydrogen production from water using a catalyst and solar energy is an ideal future fuel source. The search for suitable semiconductors as photo-catalysts for water splitting into molecular hydrogen and oxygen has been considered to be an urgent subject in our daily life. In this review, we aim to focus on the research efforts that have been made so far for H2 generation from water splitting by UV and visible-light-driven photo-catalysis. A number of synthetic modification methods for adapting the electronic structure to enhance the charge separation in photo-catalyst materials are discussed. Sacrificial reagents and electron mediators for the overall water splitting are also reviewed. The quantum efficiency of photo-catalyst materials upon visible and UV illumination will be reviewed, summarized and discussed [22].
Importance of radiations in hydrogen generation
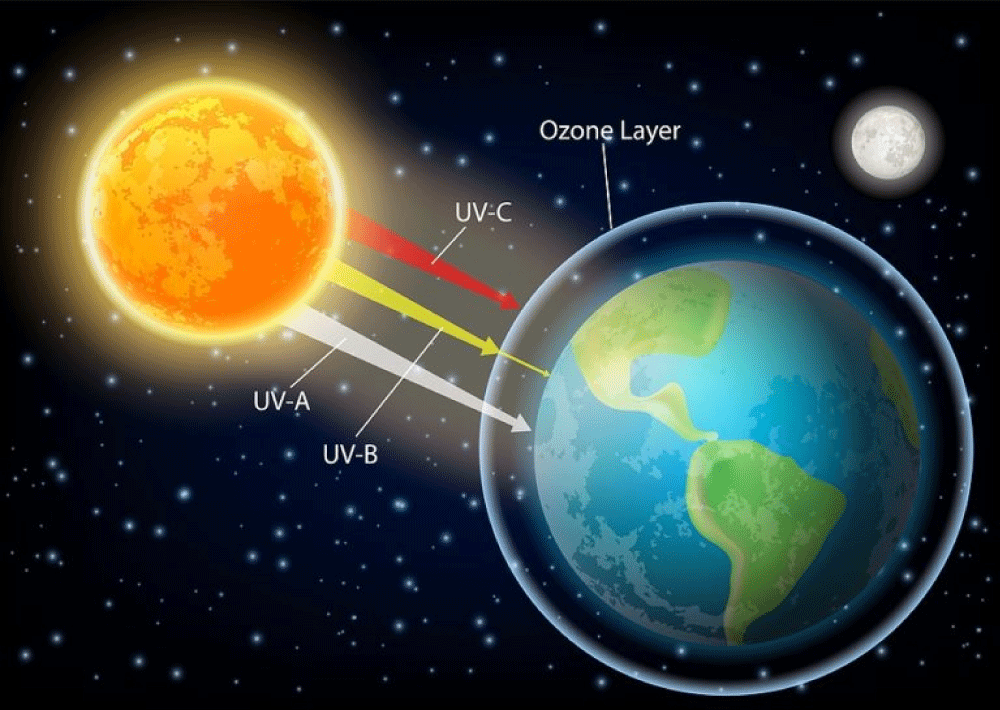
UV radiation: In the past, manufacturers’ labeling of sunscreen varied greatly, confusing consumers regarding the efficacy and the appropriate photoprotection provided by their products (Figure 4).
Figure 4: The ultraviolet component of the electromagnetic spectrum [23-24].
This article discusses ultraviolet radiation and the positive and negative effects of ultraviolet radiation provides a review of sunscreens and discusses the new regulations for sunscreens of the United States Food and Drug Administration (FDA) [23,24].
Visible radiation: Energy radiated in the visible part of the spectrum of various oxides and their mixtures when heated to red brightness temperatures between 1400 and 2000K by means of cathode-ray bombardment, and gas-air and oxy-gas flames were measured by an optical pyro-metric method. [25,26]. The energy of this radiation must be captured as excited electron-hole pairs in a semiconductor, a dye, or a chromophore, or as heat in a thermal storage medium (Figure 5).
Figure 5: The different ways of utilizing the energy coming from solar and ultraviolet irradiation [26-27].
Materials, for thermal applications, have optical properties that make them well adapted for utilizing solar energy and for reaching energy efficiency, especially in the built environment [27].
Gamma radiation: The kinetics of molecular hydrogen accumulation at gamma radiolysis of water on n-MeO surface is investigated. The influence of gamma radiations on n-MeO+water systems are studied at various temperatures (Table 1) T = 373…673 K. [28-32].
| Table 1: The value of the rates and radiation-chemical yields of molecular hydrogen during radiation-thermal, thermal and radiation processes of water decomposition in the n-ZrО2+Н2Оabs. the system at different temperatures with different particle sizes. | |||||
| Т, K | Particle size d, nm | WRT(Н2), molecules/g·s | WT(Н2), molecules/g·s |
WR(Н2), molecules/g·s |
G(Н2), molecules/100eV |
| 373 | 20-30 | 9,17 · 1013 | 1,38 · 1013 | 7,8 · 1013 | 4,8 |
| 473 | 2,08 · 1014 | 0,56 · 1014 | 1,52 · 1014 | 8,35 | |
| 573 | 3,33 · 1014 | 1,11 · 1014 | 2,22 · 1014 | 13,6 | |
| 673 | 6,94 · 1014 | 2,78 · 1014 | 4,16 · 1014 | 25,7 | |
| 373 | 50-70 | 7,88 · 1013 | 2,5 · 1013 | 5,28 · 1013 | 3,3 |
| 473 | 1,5 · 1014 | 0,5 · 1014 | 1,0 · 1014 | 6,2 | |
| 573 | 2,78 · 1014 | 0,83 · 1014 | 1,95 · 1014 | 10,4 | |
| 673 | 5,14 · 1014 | 2,08 · 1014 | 3,06 · 1014 | 19,6 | |
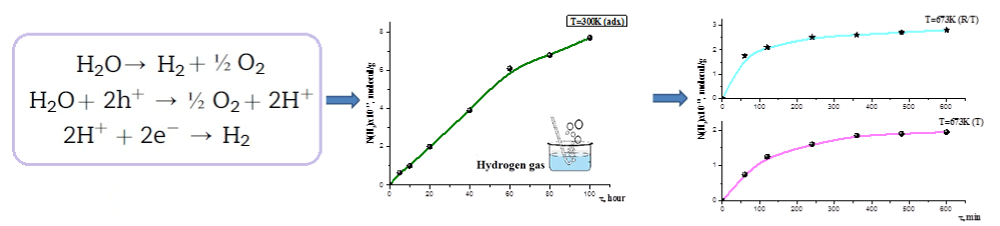
It is found that n-MeO displays radiation-catalytic activity in the decomposition of hexane and hexane–water mixture, as a result of which the rate of accumulation of molecular hydrogen increases (Figures 6,7).
Figure 6: Kinetics of the formation of molecular hydrogen during the radiation-heterogeneous decomposition of water in the systems n-MeO + Н2О [28].
Figure 7: Gamma radiation. These results are promising for molecular hydrogen generation by water splitting in near future [33-34].
These results are promising for molecular hydrogen generation by water splitting in near future [33,34].
X-ray radiation: It is desirable that the employed catalyst is nano-sized and disperses throughout the system to increase catalyzing efficiency [35-39].
The formation of radiolytic (Figure 8) products such as H2 or H2O2 has to be evaluated for safety reasons, in order to prevent the breaking or the corrosion of the confining matrix [40-44].
Figure 8: X-ray radiation and Family tree of hydrogen storage materials [35-40].
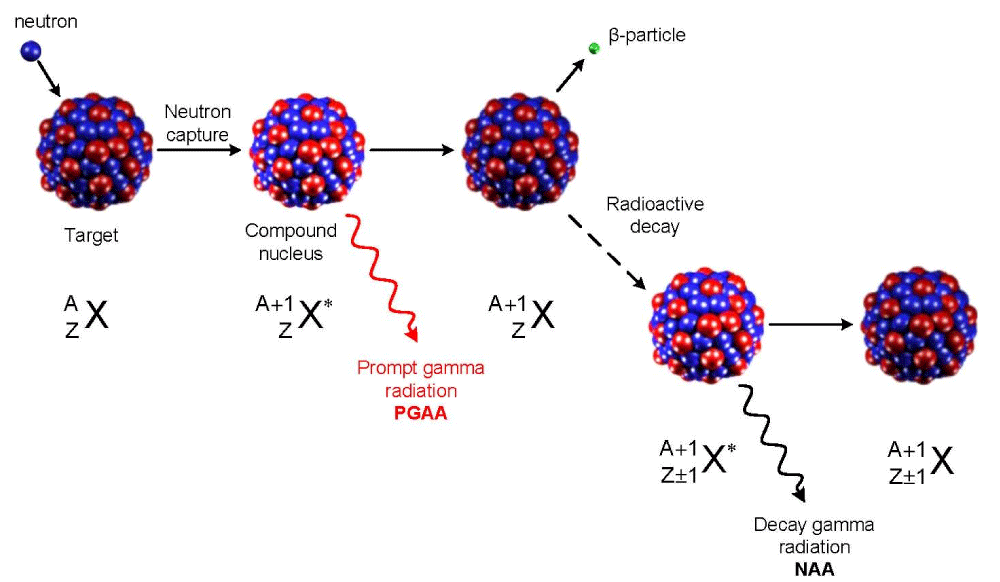
Neutron radiation: Hydrogen saturation of titanium-based materials exposed to irradiation with resonance neutrons with an energy of 0.1 MeV is considered [44,45] (Figure 9).
Figure 9: Neutron radiation [43-46].
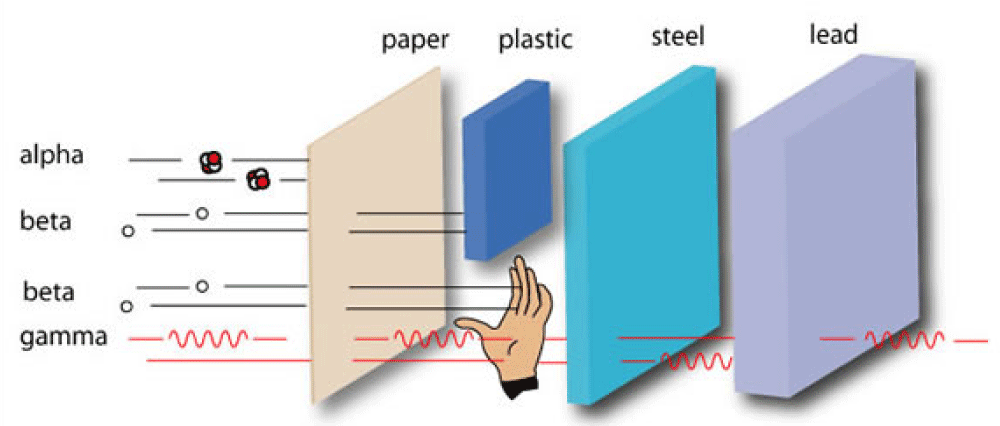
Shielding neutrons involve three steps that are slowing neutrons, absorption of neutrons, and impregnation of gamma rays. Neutrons slow down with thermal energy by hydrogen, water, paraffin and plastic. Hydrogenated materials are also very effective for the absorption of neutrons [46].
Mechanism of radiation in hydrogen generation
Electrons generated by radiation inside a nanoparticle of radius R suspense in fluid water are diffused with equal probability in all directions inside the particle and gradually lose their kinetic energy as a result of elastic and inelastic collisions. Some of these electrons are transported to the nanoparticle surface during the physical and physicochemical stages of the process and emitted into the water. Using the Monte Carlo, step-by-step, single-collision methods, the trajectories of electrons were tracked based on the Mathcad program and the percentage of electron emissions from the nanoparticle surface into the water was calculated. It was found from the calculations that the emission percentage changes depending on the size of the nanoparticle and the energy of the electrons [47-51].
Future perspective of radiations role in hydrogen generation
Herein in this review, the results of exploring the prospects of the hydrogen economy are presented. It is shown that the comparative analysis of the economic competitiveness of these chains with each other with solutions based on the use of alternative fuels has been performed, respectively. The analysis has established the most promising directions in the development of the hydrogen economy in order to justify the economical value of this research.
The monitoring of different radiation-induced changes in the surface and the choice of the performance characteristic of a heat-resistant catalytic material based on these nanomaterials. Thus, in this way, the base of nanomaterials’ radiation-catalytic processes enables the transformation of ionizing radiation energy to chemical one with great efficiency. Radiation-heterogeneous systems with nano-size open a new actual direction in radiation processes and the radiative study of materials. It is clear from the presented results that nanostructured materials become an important role in nuclear-power engineering as structural and functional materials practically in all stages of the nuclear fuel cycle. It is extremely important for the formation of nanostructured materials after exposure to ordered nanostructure from the new phase with a period of a few nanometers, promoting the preservation of the properties of materials under high irradiation.
- Balgude SD, Barkade SS, Mardikar SP. Metal Oxides for High-Performance Hydrogen Generation by Water Splitting,taylorfrancis, eBook ISBN 9780429296871. 2020; 26.
- Souza LD, Thermochemical hydrogen production from water using reducible oxide materials: a critical review, Materials for Renewable and Sustainable Energy. 2013; 2:7.
- LaVerne JA. Hydrogen Production in the Radiolysis of Dodecane and Hexane, Solvent Extraction and Ion Exchange. 2017; 35:3; 210-220.
- La Verne JA, and Tandon L. H2 Production in the Radiolysis of Water on CeO2 and ZrO2, J. Phys. Chem. B. 2002; 106:2; 380-386.
- Chang Z, Laverne JA. Hydrogen production in γ‐ray and helium‐ion radiolysis of polyethylene, polypropylene, poly(methyl‐methacrylate), and polystyrene, John Wiley & Sons, Inc. J PolymSci A: PolymChem. 2000; 38:1656-1661.
- Kalanur SS. HyungtakSeo, Electrocatalysts for Photochemical Water-Splitting, Methods for Electrocatalysispp. 2000; 171-199.
- Abe R. Development of a new system for photocatalytic water splitting into H2 and O2 under visible light irradiation. BCSJ. 2011; 84:1000-1030.
- Badwal SPS, Giddey S, Munnings C, Hydrogen production via solid electrolytic routes. Wiley Interdisc Rev Energy Environ. 2013; 2:473-487.
- Bard AJ, Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. J Photochem. 1979; 10:59-75.
- Cao S, Wang CJ, Lv XJ, Chen Y, Fu WF, A highly efficient photocatalytic H2 evolution system using colloidal CdSnanorods and nickel nanoparticles in water under visible light irradiation. ApplCatal B. 2015; 162:381-391.
- Chen S, Takata T, Domen K. Particulate photocatalysts for overall water splitting. Nat Rev Mater. 2017; 2:17050.
- Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972 Jul 7;238(5358):37-8. doi: 10.1038/238037a0. PMID: 12635268.
- Holladay JD, Hu J, King DL, Wang Y. An overview of hydrogen production technologies. Catal Today. 2009; 139: 244-260.
- Reddy VR, Hwang DW, Lee JS, Photocatalytic water splitting over ZrO2 prepared by precipitation method. Korean J Chem Eng. 2003; 20:1026-1029.
- Sayed FN, Jayakumar OD, Sasikala R, Kadam RM, Bharadwaj SR, Kienle L, Schürmann U, Kaps S, Adelung R, Mittal JP, Tyagi AK, Photochemical hydrogen generation using nitrogen-doped TiO2–Pd nanoparticles: facile synthesis and effect of Ti3+ incorporation. J Phys Chem C. 2012; 116:12462-12467.
- Yoshida M, Takanabe K, Maeda K, Ishikawa A, Kubota J, Sakata Y, Ikezawa Y, Domen K. Role and function of noble-metal/Cr-layer core/shell structure cocatalysts for photocatalytic overall water splitting studied by model electrodes. J Phys Chem C. 2009; 113:10151-10157.
- Zong X, Yan H, Wu G, Ma G, Wen F, Wang L, Li C. Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as Cocatalyst under visible light irradiation. J Am Chem Soc. 2008 Jun 11;130(23):7176-7. doi: 10.1021/ja8007825. Epub 2008 May 13. PMID: 18473462.
- Dale Wilson B, Moon S, Armstrong F. Comprehensive review of ultraviolet radiation and the current status on sunscreens. J Clin Aesthet Dermatol. 2012 Sep;5(9):18-23. PMID: 23050030; PMCID: PMC3460660.
- Zeman G. Ultraviolet Radiation, Health Physics Society, ScD, CHP. 1987; 90-98.
- Hartwig HG, Veen TV. Spectral characteristics of visible radiation penetrating into the brain and stimulating extraretinal photoreceptors, journal of comparative physiology. 1979; 130:277-282.
- PhillipsML. Visible Radiation Characteristics of Incandescent Oxides, Phys. Rev. 1928; 32:832.
- SinghV, RayalI, SharmaH. Solar radiation and light materials interaction,Energy Saving Coating Materials. 2020; 1-32.
- Imanova GT, Agayev TN, Jabarov SH. Investigation of structural and optical properties of zirconia dioxide nanoparticles by radiation and thermal methods, Modern Physics Letters B. 2021; 2150050-14.
- I Ali, Imanova GT, Garibov AA, Agayev TN, Jabarov SH, Almalki ASA, Alsubaie A. Gamma rays mediated water splitting on nano-ZrO2 surface: Kinetics of molecular hydrogen formation, Radiation Physics and Chemistry. 2021; 109431.
- Imanova GT, Hasanov SH. Observation the initial radiation to the surface of mixed nano catalyst on oxidation processes, International Journal of Scientific and Engineering Research. 2020; 869-873.
- Imanova GT. Gamma Rays Mediated Hydrogen Generation By Water Decomposition On Nano-ZrO2 Surface, Modern Approaches on Material Science. 2021; 508-514.
- Agayev TN, Musayeva ShZ, Imanova GT. Studying the Kinetics of Formation of Molecular Hydrogen during the Radiolysis of Hexane and a Mixture of C6H14–H2O on a Surface of n-ZrO2, Russian Journal of Physical Chemistry A. 2021; 270-272.
- Imanova GT. Kinetics of Radiation-Heterogeneous and Catalytic Processes Of Water In The Presence Of Zirconia Nanoparticles, Advanced Physical Research. 2020; 94-101.
- Imanova GT, Agaev TN, Garibov AA, Melikova SZ, Jabarov SH, Akhundzada HV. Radiation-thermocatalytic and thermocatalytic properties of n-ZrO2-n-SiO2 systems in the process of obtaining hydrogen from water at different temperatures, Journal of Molecular Structure. 2021; 130651.
- Imanova GT, KayaM. Importance of the Radiations in Radiolysis Processes for Hydrogen Generation, Book - Generis publishing. 2021;ISBN: 978-1-63902-693-7:50.
- Ali I, Gunel T, Imanova X, Mbianda Y, Omar ML. Alharbi, Role of the radiations in water splitting for hydrogen generation, Sustainable Energy Technologies and Assessments. 2022; 101926.
- Imanova G. Molecular hydrogen production by radiolysis of water on the surface of nano-ZrO2 under the influence of gamma rays, Synthesis and Sintering. 2022; 2:9-13.
- Akdemir M, Karakas DE, Gunel T. Imanova, Hilal Demir Kivrak, Mustafa Kaya, High Efficiency Biomass-Based Metal-Free Catalyst as a Promising Supercapacitor Electrode for Energy Storage, SSRN Electronic Journal, Elsevier publishing. (2021).
- Mahmudov H, Suleymanov T, Sabzaliyeva Z, Imanova G, Akhundzada H, Azizova K, Hasanova S, Hasanov S. Kinetic Interaction of Hexan Conversion and Oxidation on the Surface of an Al2O3 Nanocatalyzer at Room Temperature under the Effect of Gamma Radiation, Hindawi publishing, Journal of Chemistry. 2021; 9493765:6.
- Barkaoui S, Imanova G. Hydrothermal Synthesis of Co3O4 Urchin-Like and their Catalytic Properties in Co Oxidation, Juniper Online Journal Material Science (JOJMS). 2022; 7(1):555704.
- Ali I, Imanova G. Sorbtion: A Universal Technology For Water Purfication, Advanced Physical Research. 2022; 4:1; 5-9.
- Barkaoui S, Chakhari S, Kouass S, Dhaouadi H, Imanova G, Touati F. Influence of Ag-doping-cobalt oxide on the structure, optical properties, morphology and preferential oxidation activity of CO, Advanced Physical Research. 2022; 4:1; 22-32.
- Cheng H, Lu C, Liu J, Yan Y, Han X. Synchrotron radiation X-ray powder diffraction techniques applied in hydrogen storage materials, Progress in Natural Science: Materials International. 2017; 27:1; 66-73.
- Shao H, Xin G, Zheng J, Li X, Akiba E. Nano Energy. 2012; 1:590-601.
- Zheng J, Liu X, P. Xu, P. Liu, Y. Zhao, J. Yang, Int. J. Hydrog. Energy. 2012; 37:1048-1057.
- Pukazhselvan D, Kumar V, Singh S. Nano Energy. 2012; 1:566-589
- Yang J, Sudik A, Wolverton C, Siegel DJ. High capacity hydrogen storage materials: attributes for automotive applications and techniques for materials discovery. Chem Soc Rev. 2010 Feb;39(2):656-75. doi: 10.1039/b802882f. Epub 2009 Sep 14. PMID: 20111786.
- Schmid TE, Dollinger G, Hable V, Greubel C, Zlobinskaya O, Michalski D, Molls M, Röper B. Relative biological effectiveness of pulsed and continuous 20 MeV protons for micronucleus induction in 3D human reconstructed skin tissue. Radiother Oncol. 2010 Apr;95(1):66-72. doi: 10.1016/j.radonc.2010.03.010. Epub 2010 Mar 26. PMID: 20347168.
- Maquille A, Jiwan JL, Tilquin B. Radiosterilization of drugs in aqueous solutions may be achieved by the use of radioprotective excipients. Int J Pharm. 2008 Feb 12;349(1-2):74-82. doi: 10.1016/j.ijpharm.2007.07.024. Epub 2007 Jul 24. PMID: 17765417.
- Guo ZB. Gamma irradiation-induced Cd2+ and Pb2+ removal from different kinds of water. Rad. Phys. Chem. 2008; 77:1021-1026.
- Katayama T. Radiation-induced polymerization of gum arabic (Acacia senegal) in aqueous solution. Food Hydrocolloid. 2006; 20:983-989.
- Bouniol P. A comprehensive model to describe radiolytic processes in cement medium. J. Nucl. Mat. 2008; 372:1-15.
- Larionov VV, Varlachev VA, Shupeng X. Accumulation of hydrogen in titanium exposed to neutron irradiation, International Journal of Hydrogen Energy. 2020; 45:30; 15294-15301.
- Haigh RC. Hydrogen and helium production in structural materials by neutrons, International Conference on Nuclear Data for Science and Technology. 2007; 1082-84.
- Uğur FA. New applications and developments in the neutron shielding, EPJ Web of Conferences. 2017; 154:01022.
- Pikaev AK. Dosimetry in radiation chemistry M: Science. 1975; 232.