Research Article
Convenient route synthesis of some new benzothiazole derivatives and their pharmacological screening as antimicrobial agents

Ahmed A Fadda*, Nanees N Soliman and Ahmed Fekri
Department of Chemistry, Mansoura University, Mansoura, Egypt
*Address for Correspondence: Ahmed A Fadda, Faculty of Science, Department of Chemistry, Mansoura University, Mansoura, Egypt, Email: [email protected]
Dates: Submitted: 10 April 2017; Approved: 28 August 2017; Published: 29 August 2017
How to cite this article: Fadda AA, Soliman NN, Fekri A. Convenient route synthesis of some new benzothiazole derivatives and their pharmacological screening as antimicrobial agents. Ann Adv Chem. 2017; 1: 032-046. DOI: 10.29328/journal.aac.1001004
Copyright License: © 2017 Fadda AA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: The reaction of 2-(benzo]d[thiazol-2-yl)-3-oxopentanedinitrile 4 with DMF/DMA has been investigated to explore the synthetic potentialities of this novel activated nitrile in heterocyclic synthesis.
Results: Pyrano, pyridino, pyrazolo, azepino and oxothiepano carbonitrile derivatives could be obtained starting from 4 and plausible mechanisms for their formations are reported.
Conclusion: The newly synthesized compounds were assessed for their antimicrobial activity. Compounds 7, 10 and 12 exhibited broad spectrum antibacterial profile against the tested organisms.
Introduction
Thiazoles and benzothiazole derivatives represent a well known important group of heterocylic compounds due to their biological and pharmaceutical activities. Benzothiazoles with cyanomethyl group at position-2 have been the subject of extensive study in the recent past. Numerous reports have appeared in the literature, which highlight their chemistry and uses.
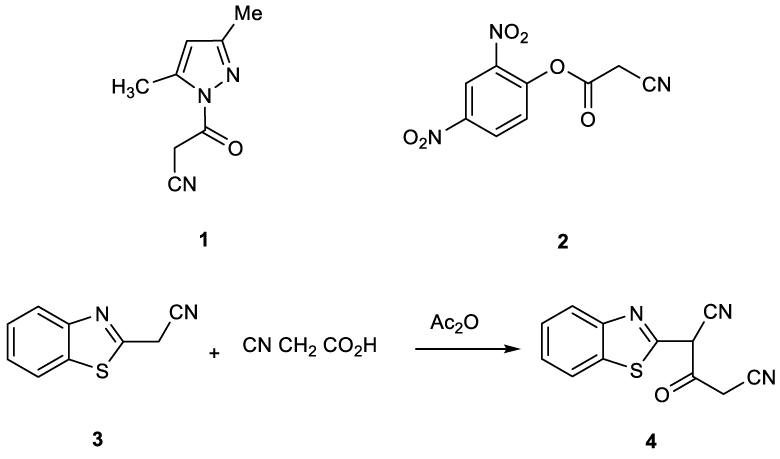
Cyanoacetylation of uracils or their derivatives [1-4], has been reported to be successfully achieved by heating the respective substrate with a mixture of acetic anhydride and cyanoacetic acid as a cyano-acetylating mixture. The structure of the reactive species formed in this case has been assigned as a cyano-ketene. The use of this cyano-acetylating mixture (cyanoacetic acid and acetic anhydride) has somehow been forgotten and on the other hand less convenient reagents like the pyrazole derivative 1 has been used [5] (Figure 1). The phenolic ester 2 of cyanoacetic acid is also suggested for cyano-acetylation since it generates cyano-ketene when heated [6]. In the last two decades, we have been involved in a program aiming to develop new simple procedures or novel precursors for the synthesis of heterocyclic compounds of biological interest to be evaluated as biodegradable agrochemicals [7-9]. In continuation with this program some heterocyclic compounds containing the benzothiazole nucleus were required for biological activity studies. Therefore, 2-(benzo]d[thiazol-2-yl)-3-oxopentanedinitrile (4) was obtained for the first time by us via cyanoacetylation of 2-cyanomethylbenzothiazole (3) seemed to be a versatile candidate to fulfill this objective (Figure 1).
Results and Discussion
In continuation of our program and following our previous interests [9-17], in the synthesis of new benzothiazole compounds of anticipated biological activity, it has been found that compound 3 will lead to an excellent building block for the synthesis of target compounds. Thus, when 2-cyanomethylbenzothiazole (3) was treated with cyanoactic acid in presence of acetic anhydride, it afforded the corresponding compound 4, as only one isolable product which indicated by TLC control tested (Figure 1). Synthesis of this compound was reported for the first time by us (Fadda et al. 2010) [10].
Moreover, benzothiazole derivative 4 has latent functional substituent which renders it to be a versatile starting substrate for further chemical transformations that open new routes for the preparation of substituted benzothiazole derivatives with possible biological activity.
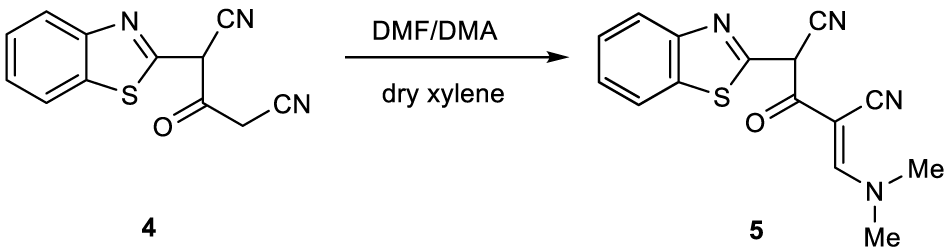
Now, we have extended our synthetic program to the synthesis of otherwise inaccessible heterocyclic ring system utilizing compound 4 as the key starting material. Thus, a mixture of 4 and DMF/DMA in dry xylene was refluxed for 5-6 hours to yield a product formulated as 5 (Figure 2). Structure 5 was illustrated based on its correct elemental and spectral analyses. The structure of compound 5 was confirmed by the 1H NMR spectrum which revealed a singlet signal at δ 2.47 ppm due to two methyl protons and a singlet signal at δ 7.60 ppm due to olefinic CH proton. Also, the mass spectrum showed a molecular ion peak at m/z 296 (M+, 80%) corresponding to the molecular formula C15H12N4OS.
Figure 2: Synthesis of 2-]Benzo]d[thiazole-2–yl[-4-((dimethylamino)methylene)-3-oxopentanedinitrile (5).
Reaction of this type has not been previously reported, however it was found to give products in excellent yield under very mild conditions. Compounds containing a pyrimidine ring have been reported to exhibit antimicrobial as well as anti-HIV activity [15,18]. On the other hand, pyran ring is known to have a broad spectrum of biological activity. Combination of the two above ring systems may be lead to very active bioactive compounds.
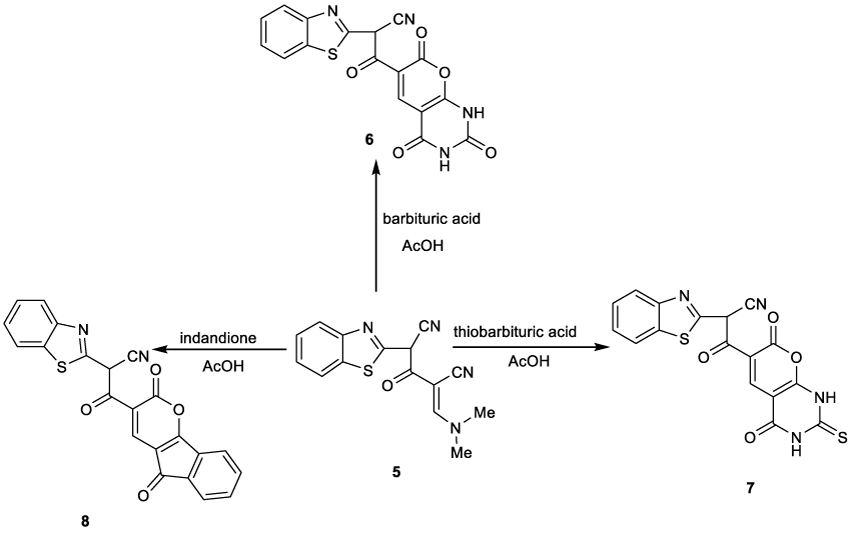
Thus, it has been found that, 2-(benzo]d[thiazole-2-yl)-4-((dimethylamino)methylene)-3-oxopentanedinitrile) (5) reacted with barbituric acid in boiling glacial acetic acid to give the corresponding 2-(benzo]d[thiazole-2-yl)-3-(2,3,4,7-tetrahydro-2,4,7-trioxo-1H-pyrano]2,3-d[pyrimidin-6-yl)-3-oxopropanenitrile (6) in 87% yield (Figure 3). Structure 6 was confirmed by both elemental and spectral data. The IR spectrum displayed the presence of an absorption band at 3350 cm-1 characteristic to NH group and 1720, 1690, 1685 cm-1 characteristic to four CO groups. The 1H NMR spectrum revealed a singlet signal at δ 8.47 ppm due to C4-H pyran and two singlet signals at δ 9.50, 10.10 ppm due to two NH groups’ protons. Also, the mass spectrum showed the molecular ion peak at m/z (%) 380 (M+, 30) corresponding to the molecular formula C17H8N4O5S.
Figure 3: Reactions of 2-]Benzo]d[thiazole-2–yl[-4-((dimethylamino)methylene)-3-oxopentanedinitrile (5) with barbituric, thiobarbituric acid and indandione.
Similarly, it was found that refluxing a mixture of compound 5with thiobarbituric acid in glacial acetic acid afforded 2-(benzo]d[thiazol-2-yl)-3-(2,3,4,7-tetrahydro-4,7-dioxo-2-thioxo-1H-pyrano]2,3-d[pyrimidin-6-yl)-3-oxopropanenitrile (7) (Figure 3). The structure was confirmed by elemental and spectral analyses. The 1H NMR spectrum revealed a singlet signal at δ 8.49 ppm due to C4-H pyran and two singlet signal at δ 10.10, 11.20 ppm due to two NH groups’ protons. Also, the mass spectrum showed the molecular ion peak at m/z (%) 396 (M+, 80) corresponding to the molecular formula C17H8N4O4S2.
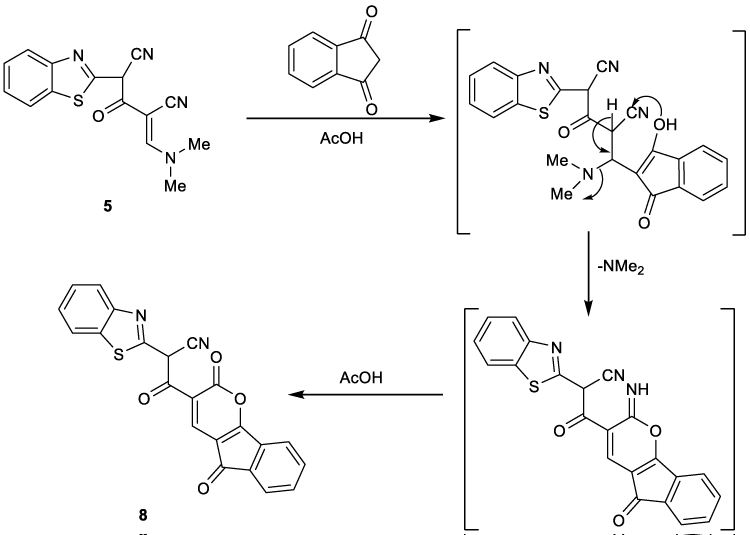
In the same manner, compound 5 was reacted with 1,3-indandione in boiling glacial acetic acid to give the corresponding indenopyranone derivative 8 (Figure 3). 1H NMR revealed two singlet signals at δ 4.64 and 8.47 ppm due to methine proton and C4-H pyran ring. The appearance of signal of C4-H pyranone ring in this low magnetic field (δ 8.47 ppm) is obviously due to the deshielding effect by the adjacent carbonyl group on the indinone, supporting its correct structure 8, unambiguously. Also, the mass spectrum showed the molecular ion peak at m/z (%) 398 (M+,70) corresponding to the molecular formula C22H10N2O4S.
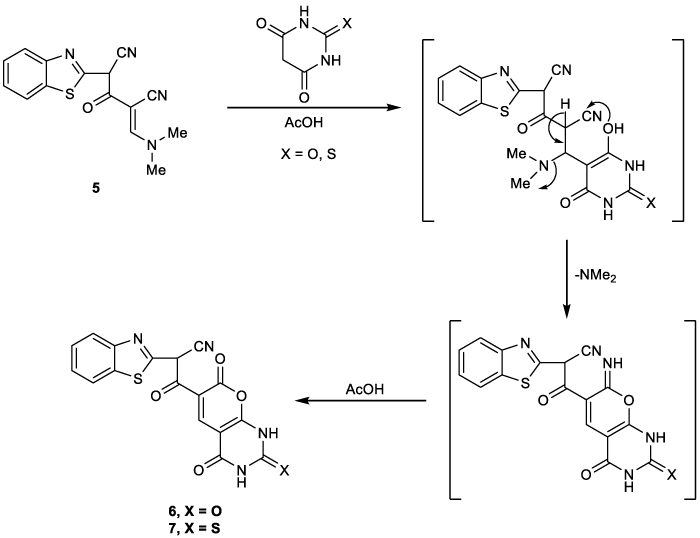
Figure 4 showed that 2-(benzo]d[thiazole-2-yl)-4-((dimethylamino)methylene)-3-oxopentanedinitrile) (5) reacted with barbituric acid X=O to give compound 6 and with thiobarbituric acid X=S to give compound 7. To account for the formation of figure (4), it is suggested that the reaction start with Michael-type addition of the CH2 group in barbituric and thiobarbituric acids to the activated double bond of compound 5 followed by elimination of dimethylamine and addition cyclization of the OH group to the nitrile group.
In the manner, Figure 5 showed that compound 5 reacted with 1,3-indandione as nucleophilic attack to CN group followed by cyclization to give indenopyranone derivative 8.
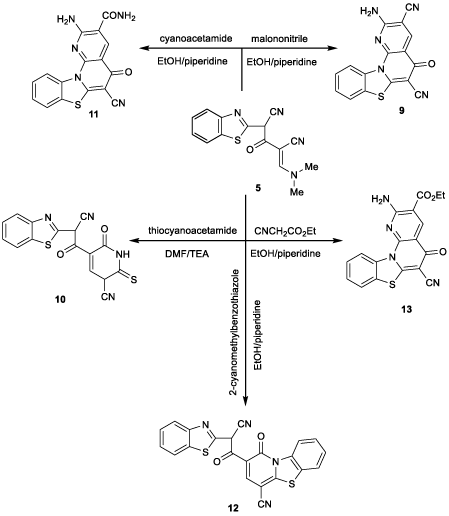
It was found that compound 5 was reacted with some activated nitriles to give benzothiazoloazepine and (benzothiazolyl) pyridine derivatives. Therefore, when 5 was reacted with malononitrile in refluxing ethanol containing a catalytic amount of piperidine it afforded the corresponding derivative 9 (Figure 6). The structure of compound 9 was confirmed by the 1H NMR spectrum which revealed a singlet signal at δ 6.49 ppm due to NH2 protons and a singlet signal at δ 8.00 ppm due to CH proton. Also, the mass spectrum showed the molecular ion peak at m/z (%) 317 (M+, 100) corresponding to the molecular formula C16H7N5OS.
Similarly, compound 5 was reacted with thiocyanoacetamide in refluxing DMF containing a catalytic amount of TEA for 6 hours. It afforded the corresponding pyridine derivative 10 (Figure 6). 1H NMR revealed spectrum singlet signal at δ 4.64 due to methine proton, doublet at δ 5.20 ppm due to C5-H pyridine ring and doublet signal at δ 7.86 ppm due to C4-H pyridine ring, singlet signal at δ 10.1 ppm due to NH proton. Also, the mass spectrum showed the molecular ion peak at m/z (%) 352 (M+, 40) corresponding to the molecular formula C16H8N4O2S2.
In a similar reaction mechanism to that of compound 9, compound 5 reacted with cyanoacetamide to afford compound 11 (Figure 6). The mass spectrum showed the molecular ion peak at m/z (%) 335 (M+,50) corresponding to the molecular formula C16H9N5O2S.
On the other hand, compound 5 was reacted with (2-cyanomethyl) benzothiazole in refluxing ethanol containing a catalytic amount of piperidine, it afforded the corresponding benzothiazolopyridine derivative 12 (Figure 6). The 1H NMR spectrum showed two singlet signals at δ 4.65 and 7.86 ppm attributable to methine and C4-H pyridine protons, respectively. Also, the mass spectrum showed the molecular ion peak at m/z (%) 426 (M+, 50) corresponding to the molecular formula C22H10N4O2S2.
In a similar reaction mechanism to that of compound 9, compound 5 was reacted with ethyl cyanoacetate in refluxing ethanol containing a catalytic amount of piperidine for 8 hours. It afforded derivative 13 (Figure 6). The 1H NMR spectrum showed triplet signal at δ 1.38 ppm due to CH3 protons, quartet signal at δ 4.35 ppm due to CH2 protons and a singlet signal at δ 6.50 ppm due to NH2 protons.
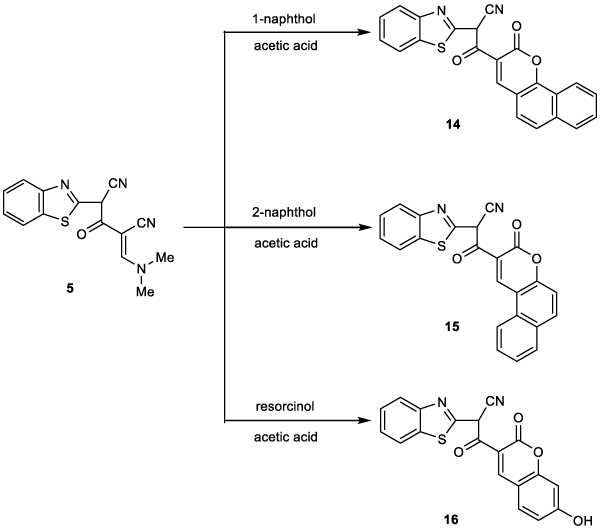
In addition, heating 5 with an equimolar amount of α-naphthol in refluxing glacial acetic acid gave the corresponding benzocoumarine derivative 14 (Figure 7). The 1H NMR spectrum showed a singlet signal at δ 4.65 ppm due to CH proton, doublet signal at δ 7.21 ppm due to aromatic protons, multiplet signal at δ 7.35-8.23 ppm due to aromatic protons and a singlet signal at δ 8.45 ppm due to C4-H pyran. The mass spectrum showed the molecular ion peak at m/z (%) 396 (M+,50) corresponding to the molecular formula C23H12N2O3S.
Similarly, compound 5 reacted with β-naphthol to give the corresponding benzocoumarine derivative 15 (Figure 7). The IR and 1H NMR spectra showed a similar picture to that of compound 14. Heating compound 5 with resorcinol in glacial acetic acid afforded the corresponding 7-hydroxycoumarine derivative 16 (Figure 7). The 1HNMR spectrum showed four singlet signal at δ 4.65, 5.00, 7.10 and 8.27 ppm due to C-H (methine proton), OH, C4-H and CC8-H coumarine ring, respectively. Besides two doublets at δ 6.55 and 7.90 ppm due to aromatic protons and multiplet signals at δ 7.55-8.23 ppm due to the other aromatic protons. The mass spectrum showed the molecular ion peak at m/z (%) 362 (M+,40) corresponding to molecular formula C19H10N2O4S.
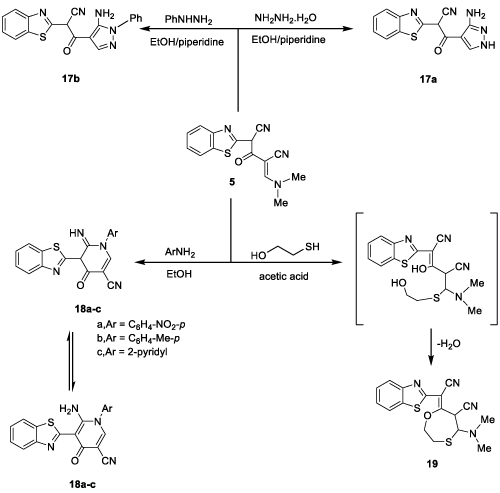
Moreover, it has been studied the reaction of compound 5 with some nitrogen nucleophiles which afforded a product of nucleophilic substitution reactions with N(CH3)2 moiety. Thus, it has been found that reaction with hydrazine hydrate and/or phenylhydrazine afforded the corresponding pyrazolyl derivatives 17a,b, respectively (Figure 8). The IR spectrum, in general, showed stretching frequencies at 3420, 3350, 2220, 1700 and 1640 cm-1 due to NH2, NH, CN, C=O and C=N, respectively. 1HNMR spectrum (in general) showed singlet signals at δ 4.62, 5.60 and 10.20 ppm due to CH, NH2 and NH protons while the aromatic protons appeared as multiplet at δ 7.40-7.80 ppm. The mass spectrum of 17a showed the molecular ion peak at m/z (%) 283 (M+,50) corresponding to molecular formula C13H9N5OS, while the mass spectrum of 17b showed the molecular ion peak at m/z (%) 359 (M+,60) corresponding to molecular formula C19H13N5OS.
Moreover, reaction of 5 with some aromatic amines (namely, p-nitroaniline, p-toluidine and 2-aminopyridine) in refluxing ethanol in the presence of a catalytic amount of TEA afforded the corresponding (benzothiazolyl) pyridine derivatives 18a-c (Figure 8). Structures of 18a-c were based on their correct analytical and spectral analyses. The mass spectrum of 18a showed the molecular ion peak at m/z (%) 389 (M+,80) corresponding to molecular formula C19H11N5O3S. The mass spectrum of 18b showed the molecular ion peak at m/z (%) 358 (M+,40) corresponding to molecular formula C20H14N4OS. The mass spectrum of 18c showed the molecular ion peak at m/z (%) 345 (M+,70) corresponding to molecular formula C18H11N5OS.
On the other hand, refluxing of compound 5 with mercaptoethanol in glacial acetic acid gives the cyclo addition product 19 (Figure 8). The 1H NMR spectrum revealed two methyl protons at δ 2.27 ppm as singlet signal, two triplets at δ 3.68 and 3.70 ppm due to S-CH2 and O-CH2 protons, respectively, two doublets at 2.76 and 2.86 ppm due to S-CH and C-CH protons, respectively. The mass spectrum of 19 showed the molecular ion peak at m/z (%) 356 (M+,70) corresponding to molecular formula C17H16N4OS2.
Biological activity
Antimicrobial Evaluation: Nineteen of the newly synthesized targeted compounds were evaluated for their in vitro antimicrobial activity against Bacillus subtilis and Bacillus thuringiensis as example of Gram positive bacteria and Escherichia coli y and Pseudomonas aeruginosa as example of Gram negative bacteria. They were also evaluated for their in vitro antifungal potential against Fusarium oxysporum and Botrytis fabae fungal strains.
Agar-diffusion method [19], was used for the determination of the antibacterial and antifungal activity. Chloramphenicol, Cephalothin and Cycloheximide and Ampicillin were used as reference drugs. The results were recorded for each tested compound as the average diameter of inhibition zones (IZ) of bacterial or fungal growth around the disks in mm. The minimum inhibitory concentration (MIC) measurement was determined for compounds showed significant growth inhibition zones (>14 mm) using two fold serial dilution method [20].
The MIC (μg/mL) and inhibition zone diameters values are recorded in table 1. The results depicted in table 1 revealed that the most of tested compounds displayed variable inhibitory effects on the growth of the tested Gram-positive and Gram-negative bacterial strains, and also against anti-fungal strain.
| Table 1: The minimum inhibitory concentration (MIC,μg/mL) and inhibition zone (mm) of some new synthesized compounds. | ||||||
| Compound No. | MICa in µg/mL, and inhibition zone (mm) | |||||
| Bacteria | Fungi | |||||
| Gram-positive bacteria | Gram-negative bacteria | |||||
| B. subtilis | B. thuringiensis | E. coli | P. ueruginosa | F. oxysporum | B. fabea | |
| 3 | 100(15) | 50(15) | 100(16) | 100(15) | 100(15) | 100(14) |
| 4 | 50(31) | 25(30) | 25(33) | 25(31) | 50(15) | 50(14) |
| 5 | 25(33) | 25(31) | 25(30) | 25(31) | 50(14) | 50(14) |
| 6 | 6.25(37) | 12.5(38) | 25(30) | 25(31) | 50(15) | 50(20) |
| 7 | 3.125(45) | 6.25(38) | 50(15) | 50(20) | 6.25(37) | 6.25(38) |
| 8 | 12.5(35) | 12.5(38) | 50(23) | 50(21) | 12.5(22) | 50(18) |
| 9 | 25(30) | 50(25) | 100(20) | 100(18) | 50(15) | 50(18) |
| 10 | 3.125(44) | 6.25(39) | 25(30) | 25(31) | 6.25(38) | 6.25(38) |
| 11 | 25(31) | 50(29) | 50(22) | 25(20) | 50(15) | 50(18) |
| 12 | 3.125(42) | 6.25(38) | 6.25(36) | 6.25(35) | 6.25(41) | 6.25(40) |
| 13 | 25(30) | 50(28) | 25(25) | 25(30) | 50(20) | 50(20) |
| 14 | 25(30) | 25(30) | 50(18) | 100(18) | 100(14) | 100(14) |
| 15 | 25(33) | 25(31) | 50(19) | 100(18) | 100(15) | 100(14) |
| 16 | 12.5(35) | 12.5(37) | 25(33) | 25(33) | 12.5 | 25(20) |
| 17a | 12.5(37) | 12.5(31) | 50(18) | 100(20) | 100(18) | 50(22) |
| 17b | 50(35) | 50(30) | 50(19) | 100(19) | 50(14) | 50(20) |
| 18a | 6.25(38) | 12.5(37) | 100(15) | 100(20) | 25(20) | 25(25) |
| 18b | 6.25(39) | 12.5(37) | 50(20) | 50(18) | 50(14) | 50(15) |
| 18c | 6.25(38) | 12.5(37) | 12.5(09) | 50(18) | 50(14) | 50(15) |
| Chloramphenicol | 3.125(44) | 3.125(44) | 6.25(37) | 6.25(38) | b | b |
| Cephalothin | 6.25(36) | 6.25(37) | 6.25(38) | 6.25(37) | b | b |
| Cycloheximide | B | b | b | b | 3.125(43) | 3.125(40) |
| Ampicillin | 3.125(40) | b | 6.25(38) | b | b | b |
| aMIC: Minimum inhibitory concentration values with SEM= 0.02 (the lowest concentration that inhibited the bacterial growth). bNT: Not tested |
||||||
On the other hand, compounds 5, 9,11,14,15 exhibited moderate growth inhibitory against Gram-positive bacteria as revealed from MIC values (25μg/mL). Compound 17a showed relatively good growth inhibitory profile against Bacillus subtilis was about 25% of the activity of Chloramphenicol and Ampicillin and 50% of Cephalothin against the same organism. Also, compound 17b showed weak growth inhibitory against Bacillus subtilis (MIC 50μg/mL). Regarding to the activity of the newly synthesized compounds, against antifungal strains, the results revealed that compounds 10 and 7 were 50% lower than Cycloheximide in inhibitory the growth of B. fabae and F. oxysporum (MIC 6.25μg/mL). While the activity of compound 8 and 16 were 25% lower than Cycloheximide against F. oxysporum (MIC 12.5μg/mL). It is worth mentioning that the incorporation of coumarin or benzocoumarin moiety to benzothiazole nucleus decreased the antimicrobial activity. On the other hand, conversion of compound 3 to pyridine and pyrimidine thione enhanced the antimicrobial activity. In conclusion, the objective of the present study was to synthesize and investigate the antimicrobial activities of some new functionalized benzothiazole derivatives with the hope of discovering new structure leads serving as antimicrobial agents. Our aim has been verified by the synthesis of different groups of structure hybrids comprising basically the benzothiazole moiety.
Experimental
All melting points are recorded on Gellenkamp electric melting point apparatus. The spectra ν (cm-1) (KBr) were recorded on a Perkin Elmer Infrared Spectrophotometer Model 157. The 1H NMR spectra were obtained on a Varian Spectrometer at 200 MHz, using TMS as an internal reference and DMSO-d6 as solvent. The 13C NMR spectra were recorded on JEOL-ECA500 (National Research Center, Egypt). The mass spectra were recorded at 70 eV with kratos MS equipment and/or a Varian MAT 311 A Spectrometer. Elemental analysis (C, H and N) were carried out at the Micro analytical Center of Cairo University, Giza, Egypt.
Synthesis of 2-(benzo[d]thiazol-2-yl)-3-oxopentanedinitrile (4). It was prepared according to the reported work [13].
Synthesis of 2-]benzo]d[thiazol-2–yl[-4-((dimethylamino)methylene)-3-oxopentanedinitrile (5). A solution of 4 (2.41 g , 0.01 mol) and DMF/DMA ( 1.19 ml , 0.01 mole ) in dry xylene (30 mL) the reaction mixture was refluxed for 5-6 h, then left to cool and poured into ice cold water. The formed precipitate was filtered off, dried and recrystallized from EtOH to afford compound 5; yield 70%; mp > 310°C; yellow powder; IR (KBr) (ν/cm-1): 2220, 2215(2CN), 1700(C=O), 1640(C=N), 1600(C=C); 1H-NMR (DMSO-d6) δ (ppm): 2.47(s, 6H, 2CH3) ,4.64(s,1H,CH), 7.50-8.22 (m, 4H, Ar-H), 7.60 (s, 1H, olefinic CH); 13C-NMR (DMSO-d6) δ (ppm): 43.8,44.4,81.4,115.7,118.3,121.8,124.5,125.3,135.2,152.8,154.9,166.7; MS (EI,70 eV): m/z (%) 296 (M+, 80), 269 (50), 175 (50),146 (50). Anal. Calcd for C15H12N4OS (296.35): C, 60.79; H, 4.08; N, 18.91%. Found: C, 60.65; H, 3.99; N, 18.75%.
Synthesis of 2-(benzo]d[thiazol-2-yl)-3-(2,3,4,7-tetrahydro-2,4,7-trioxo-1H-pyrano]2,3-d[pyrimidin-6-yl)-3-oxopropanenitrile (6). A mixture of 5 (2.96g , 0.01 mol), barbituric acid (1.28g, 0.01 mol) in boiling glacial acetic acid (30mL) .The reaction mixture was refluxed for 7-8h, then allowed to cool and poured into ice cold water. The obtained precipitated solid was filtered off, dried and recrystallized from EtOH to furnish compound 6; yield 87%; mp>310°C, reddish brown powder; IR (KBr) (ν/cm-1): 3350(NH), 2220(CN), 1720, 1690, 1685 (4CO); 1H-NMR (DMSO-d6) δ (ppm): 4.64 (s, 1H, CH), 7.55-8.23 (m, 4H, Ar-H), 8.47 (s, 1H, C4-Hpyran ), 9.50(s,1H,NH),10.10(s,1H,NH); 13C-NMR(DMSO-d6)δ(ppm): 45.3,87.2,115.7,121.8, 124.5,125.3,135.2,150.6,152.8,153.6,156.6,162.8,166.7,196.5; MS (EI, 70 eV): m/z (%) 380 (M+,30) ,295 (70),209(60),197(30),180 (50),166(35),153(40). Anal. Calcd for C17H8N4O5S (380.33): C, 53.68; H, 2.12; N, 14.73%. Found: C, 53.47; H, 2.01; N, 14.62%.
Synthesis of 2-(benzo]d[thiazol-2-yl)-3-(2,3,4,7-tetrahydro-4,7-dioxo-2-thioxo-1H-pyrano]2,3-d[pyrimidin-6-yl)-3-oxopropanenitrile (7). Refluxing of a mixture of 5 (2.96g, 0.01 mol) and thiobarbituric acid (1.44g, 0.01 mol) for 5 h in boiling glacial acetic acid (15mL). The reaction mixture was left to cool at room temperature, then poured into ice cold water. The obtained solid product was filtered off, dried and recrystallized from EtOH affording compound 7; yield 76%; mp>320°C, reddish brown powder; IR (KBr) (ν/cm-1):3350 (NH), 2220(CN),1730,1655(2C=O),1310 (C=S); 1H-NMR (DMSO-d6) δ (ppm):4.64 (s,1H, CH), 7.55-8.23 (m,4H,Ar-H), 8.49 ( s,1H,C4-H pyran ),Z0.10 (s,1H,NH),11.20 (s,1H,NH); 13C-NMR (DMSO-d6)δ(pm):45.3,89.7,115.7,121.8,124.5,125.3,135.2,152.5,156.6,161.5,162.8,166.9,170.1,196.5. MS (EI,70 eV):m/z (%) 396 (M+,80),366(30),305(10),212(25),184(30),168(20),155(25),139(30),128(35). Anal. Calcd for C17H8N4O4S2 (396.40): C, 51.51; H, 2.03; N, 14.13%. Found: C, 51.40; H, 1.98; N, 14.01%.
Synthesis of 2-(benzo]d[thiazol-2-yl)-3-(2,5-dioxo-2,5-dihydroindeno[1,2-b]pyran-3-yl)-3-oxopropanenitrile (8). A mixture of compound 5 (2.96g, 0.01 mol) and indandione (1.46g,0.01mol) in boiling glacial acetic acid (30mL) was refluxed for 7h. The reaction mixture was left to cool and poured into ice cold water. The precipitated solid material was filtered off dried and recrystallized from EtOH to afford compound 8; yield 70%; mp 190C; dark green powder; IR (KBr) (ν/cm-1): 2220 (CN),1700,1660,1650 (3C=O); 1H-NMR (DMSO-d6)δ (ppm):4.64 (s,1H,CH), 7.38-8.23 (m,8H,Ar-H), 8.47 (s, 1H, C4-H pyran;13C-NMR (DMSO-d6) δ (ppm): 45.3,115.7,121.8,124.5,125.3,126.2,128.4,129.7,131.8,134.7,135.2,136.5,137.6, 151.9,152.8,162.8,168.7,182.6,196.5; MS (EI,70 eV): m/z (%) 398 (M+,70), 382(40),314 (30),177(60),123 (30),107(65). Anal. Calcd for C22H10N2O4S (398.39): C, 66.33; H,2.53; N, 7.03%. Found: C, 66.13; H, 2.35; N, 6.95%.
Synthesis of 10-imino-7-oxo-7, 10-dihydrobenzo[4,5]thiazol[3,2-a]azepine-6,8,9-tricarbonitrile(9). A solution of 5 (2.96g, 0.01 mol) and malononitrile (0.066g, 0.01 mol) in boiling ethanol containing a catalytic amount of piperidine (30 mL) was refluxed for 6h. The reaction mixture was left to cool and poured into ice cold water. The solid formed was filtered off, dried and recrystallized from EtOH to afford compound 9; yield 73%; mp 215°C, black powder; IR (KBr) (ν/cm-1) : 3150 (NH), 2220(CN), 1700 (C=O), 1640 (C=N), 1560 (C=C); 1H-NMR (DMSO-d6) δ (ppm): 6.49 (s, 2H, NH2), 7.20-7.80 (m, 4H,Ar-H), 8.00 (s,1H,CH); 13C-NMR (DMSO-d6) δ (ppm): 61.8,76.0,(3) 115.8,116.5,119.0,121.1,122.9,123.7,126.3,145.6,165.6,167.9, 184.4; MS (EI, 70 eV): m/z (%) 317 (M+,100),301(50),275(30),247(20),221(20),195(25),169(25).Anal. Calcd for C16H7N5OS (317.33): C, 60.56; H, 2.22; N, 22.07%. Found: C, 60.35; H, 2.10; N, 21.98%.
Synthesis of 5-(2-(benzo]d[thiazol-2-yl)-2-cyanoacetyl)-6-imino-2-thioxo-1,2,3,6-tetrahdropyridine-3-carbonitrile (10). A mixture of 5 (2.96 g, 0.01 mol) and thiocyanoacetamide in refluxing DMF (30 mL) containing a catalytic amount of TEA for 6 h. The reaction mixture was left to cool and poured into ice cold water. The solid formed was filtered off, dried and recrystallized from EtOH to afford compound 10; yield 60%; mp 300°C; black crystals; IR (KBr) (ν/cm-1): 3320 (NH), 2219(CN), 1700, 1685 (2C=O), 1320 (C=S); 1H-NMR (DMSO-d6) δ (ppm): 4.64(s,1H,CH), 5.20(d, J=5.9 Hz, 1H, C5-H pyridine), 7.55-8.23 (m,4H,Ar-H), 7.86(d,J=6.3Hz, 1H, C4-H pyridine), 10.10(s,1H,NH); 13C-NMR (DMSO-d6) δ (ppm): 44.8,45.4,115.3, 121.6,124.5,125.3,135.2,137.7,143.2,166.7,195.6,196.5; MS(EI,70 eV): m/z (%) 352 (M+,40), 225(15), 209 (20), 184(50), 169(30), 156(70). Anal. Calcd for C16H8N4O2S2 (352.39): C, 54.69; H, 2.58; N, 19.93%. Found: C, 54.78; H, 2.44; N, 19.75%.
Synthesis of 5-(benzo]d[thiazol-2-yl)-2,3,5-tricyano-4-oxopentanamide(11). A mixture of 5 (2.96 g, 0.01 mol) and cyanoacetamide (0.84g, 0.01 mol) in refluxing ethanol (30 mL) containing a catalytic amount of piperidine for 6h. The reaction mixture was left to cool and poured into ice cold water. The solid formed was filtered off, dried and recrystallized from EtOH to afford compound 11; yield 63%; mp 130°C; IR (KBr) (ν/cm-1): 2219, 2201(2 CN), 1710, 1700 (2C=O); 1H-NMR (DMSO-d6) δ (ppm): 6.50 (s,2H,NH2) ,7.00-7.80 (m,4H,Ar-H) ,7.16 (s,2H,CONH2), 7.81 (s,1H, CH); 13C-NMR (DMSO-d6) δ (ppm): 76.0, 86.4, (2) 115.8, 116.5,119.0,121.1,122.9,123.7,126.3,145.6,159.5,165.6,172.7,184.4,187.0,MS (EI,70 eV): m/z (%) 335(M+ ,50),316(60),292(40),277(100),269(55),249(65),226(55),223(20),198(55),174(50). Anal. Calcd for C16H9N5O2S (335.34): C, 57.31; H 2.71; N, 20.88%. Found: C, 57.20; H 2.55; N, 20.75%.
Synthesis of 2-(2-(benzo]d[thiazol-2-yl)-2-cyanoacetyl)-1-oxo-1H-benzo]4,5[thiazol[3,2-a]pyridine-4-carboritrile (12). A solution of compound 5 (2.96 g, 0.01 mol) and 2-cyanomethylbenzothiazole (1.74 g, 0.01 mol) was refluxed for 6 h in absolute ethanol (20 mL) containing a catalytic amount of piperidine (4 drops). The reaction mixture was left to cool and poured into ice cold water. The precipitated solid material was filtered off, dried and recrystallized from EtOH to afford compound 12; yield 68%; mp 139°C, green powder; IR (KBr) (ν/cm-1): 2220,2219(2CN),1710,1690 (2C=O); 1H-NMR (DMSO-d6) δ (ppm): 4.65(s,1H, C-H), 7.86 (s,1H,C4-H pyridine), 7.16-8.23 (m, 8H, Ar-H), 13C-NMR (DMSO-d6) δ (ppm): 44.9,72.3, 115.8,121.8,124.5,125.3,129.0,135.2,137.1,157.5,162.9,168.7,196.5, MS (EI,70 eV): m/z (%) 426 (M+,50), 354 (40),295(35),269(20 ),234(40),192(70),175(50),163(30),147(60),133(50),121(50),107(40). Anal. Calcd for C22H10N4O2S2 (426.27): C, 61.96; H, 2.36; N, 13.14%. Found: C, 61.75; H, 2.35; N, 13.01%.
Synthesis of ethyl-5-(benzo]d[thiazol-2-yl)-2,3,5-tricyano-4-oxopentanoate(13). A solution of compound 5 (2.96 g, 0.01 mol) and ethyl cyanoacetate (1.13 g, 0.01 mol) was refluxed for 8 h in EtOH (30 mL) containing a catalytic amount of piperidine (4 drops). The reaction mixture was left to cool and poured into ice cold water. The precipitated solid material was filtered off, dried and recrystallized from EtOH to afford compound 13; yield 70%; mp 183°C; green powder; IR (KBr) (ν/cm-1): 2216, 2200 (2CN) , 1710, 1700 (2C=O); 1H-NMR (DMSO-d6) δ (ppm): 1.38 (t, J=7.2 Hz,3H, CH2CH3), 4.35 (q, J=7.2 Hz,2H,CH2CH3), 6.50 (s,2H, NH2), 7.00-7.80 (m,4H,Ar-H), 7.75 (s,1H, CH); 13C-NMR (DMSO-d6) δ (ppm): 14.2,61.8,76.0, 84.2,(2)115.8,116.5,119.0,121.1,122.9,123.7,126.3,145.6,160.7,165.0,165.6,184.4,MS (EI,70eV):m/z(%) 364(M+,70),338(60),320(30),310(25),293(25),277(90),251(50),225(30).Anal. Calcd for C18H12N4O3S (364.38): C, 59.33; H, 3.32; N, 15.38%. Found: C, 59.22; H, 3.10; N, 15.25%.
Synthesis of 2-(benzo]d[thiazol-2-yl)-3-oxo-3-(2-oxo-2H-benzo[h]chromen-3-yl)propanenitrile(14). A mixture of compound 5 (2.96g,0.01 mole) and α-naphthol (1.44g,0.01 mol) in boiling glacial acetic acid (30 mL) was refluxed for 10h. The reaction mixture was left to cool and poured into ice cold water. The solid formed was filtered off, dried and recrystallized from EtOH to afford compound 14; yield 65%; mp 145°C, black sheets; IR (KBr) (ν/cm-1): 2220 (CN),1700,1645 (2C=O); 1H-NMR (DMSO-d6) δ (ppm): δ 4.65 (s,1H, CH), 7.21 (d, J=8.4 Hz, 1H, Ar-H), 7.26 (d,J=8.9 Hz,1H,Ar-H),7.35-8.23(m,4H,Ar-H),8.45 (s,1H,C4-H pyran); 13C-NMR(DMSO-d6)δ(ppm): 45.2,115.7,117.9,120.4,121.6,122.4,124.5,125.3,126.6,127.5,131.2, 134.2,135.1,137.4,152.8,159.4,166.7,196.5,MS(EI,70 eV): m/z (%) 396(M+,50),387(100),377(50),357(30), 333(100),272(30),258(40),198(50),144(50),115(60). Anal. Calcd for C23H12N2O3S (396.42): C, 69.69; H, 3.05; N, 7.07%. Found: C, 69.47; H, 2.95; N, 6.97%.
Synthesis of 2-(benzo]d[thiazol-2-yl)-3-oxo-3-(3-oxo-3H-benzo[f]chromen-3-yl)propanenitrile (15). A mixture of compound 5 (2.96g,0.01 mole ) and β-naphthol (1.44 g,0.01 mol) in boiling glacial acetic acid (30 mL) was refluxed for 10h. The reaction mixture was left to cool and poured into ice cold water. The precipitated solid formed was filtered off, dried and recrystallized from EtOH to afford compound 15; yield 69%; mp 226°C, brown powder.’
Synthesis of 2-(benzo]d[thiazol-2-yl)-3-(7-hydro-2-oxo-3-(2-oxo-2H-chromen-3-yl)-3-oxopropanenitrile (16). A solution of compound 5 (2.96g,0.01 mol) and resorcinol (1.19g,0.01 mol) was refluxed in boiling glacial acetic acid (30 mL) for 10h. The reaction mixture was left to cool and poured into ice cold water. The solid obtained was filtered off, dried and recrystallized from EtOH to afford compound 16; yield 72%; mp > 300°C, black powder; IR (KBr) (ν/cm-1): 3450(OH),2220(CN),1710,1650(2C=O); 1H-NMR (DMSO-d6) δ (ppm):4.65(s,1H,C-H),5.00(s,1H,OH),6.55(d,J=8.1Hz,1H,C5-H),7.10 (s,1H,C4-Hpyran),7.90(d,J=7.7Hz,1H, C6-H),8.27(s,1H,C8-H),7.55-8.23 (m,4H,Ar-H);13C-NMR (DMSO-d6)δ(ppm): 45.2,102.5,110.7,112.6, 115.7, 121.6,124.5,125.3,130.2,131.2,135.2,137.4,152.8,157.3,159.4,168.7,196.5;MS(EI,70eV): m/z (%) 362 (M+, 40),359 (100),331(45),293(70),269(80),251(80),226(45),223(70),198(50),170(30),146(45). Anal. Calcd for C19H10N2O4S (362.36): C, 62.98; H, 2.78; N, 7.73%. Found: C, 62.79; H, 2.67; N, 7.53%.
Synthesis of 3-(3-amino-1-aryl-1H-pyrazol-4-yl)-2-(benzo[d]thiazol-2-yl)-3-oxopropanenitrile (17a,b).
General procedure. A mixture of 5 (0.01 mol) and hydrazine hydrate and/or phenylhydrazine (0.01 mol) in EtOH (30mL) and a catalytic amount of TEA was refluxed for 7h. The reaction mixture was allowed to cool and poured into ice cold water. The obtained precipitated solid obtained was filtered off, dried and recrystallized from EtOH to afford compounds 17a and 17b, respectively.
3-(3-Amino-1H-pyrazol-4-yl)-2-(benzo[d]thiazol-2-yl)-3-oxopropanenitrile(17a). Yield 65%; mp 200C; dark brown powder; IR (KBr) (ν/cm-1): 3420(NH2),3350(NH),2220(CN),1700(C=O),1640 (C=N); 1H-NMR (DMSO-d6) δ (ppm): 4.62 (s,1H,CH),5.60(s,2H,NH2),10.2(s, 1H, NH),7.40-7.80 (m,4H,Ar-H); 13C-NMR (DMSO-d6) δ (ppm): 45.4,97.3,115.7,121.6,121.8,124.5,125.3,135.2,137.6,152.8,153.0,168.7,190.4; MS (EI, 70 eV): m/z (%)283 (M+,50),268(70),264(50),258(90),252(40),241(40),201(70),174(75). Anal. Calcd for C13H9N5OS (283.31): C, 55.11; H,3.20; N, 24.72 %. Found: C, 55.00; H, 3.10; N, 24.61%.
3-(3-Amino-1-phenyl-1H-pyrazol-4-yl)-2-(benzo[d]thiazol-2-yl)-3-oxopropanenitrile (17b). Yield 65%; mp 135°C; brown powder; IR (KBr) (ν/cm-1): 3400, 3350(2NH), 2219, 2220(2CN), 1700(C=O). 1H-NMR (DMSO-d6) δ (ppm): 4.65(s,1H,C-H),6.71-8.23 (m, 9H, Ar-H),7.54 (s, 1H, CH=C), 9.50 (s, 1H , NH), 10.10 (s, 1H, NH), 13C-NMR (DMSO-d6) δ (ppm): 45.4,104.8,113.2,115.7,(2)119.3,121.6,121.8,124.5,125.3, 127.9,(2)129.2,135.2,149.0,152.8,163.2,168.7,190.5; MS (EI, 70 eV): m/z (%) 359 (M+, 50), 291(100),253 (70),241(60),137(60). Anal. Calcd for C19H13N5OS (359.41): C, 63.50; H, 3.65; N, 19.49%. Found: C, 63.35; H, 3.54; N, 19.36%.
Synthesis of 1-aryl-5-(benzo[d]thiazol-2-yl)-6-imino-4-oxo-1,4,5,6-tetrahydropyridine-3-carbonitrile (18a-c).
General procedure. A mixture of 5 (0.01 mol) and aromatic amines ( 0.01 mol) namely p-nitroaniline, p-toluidine and 2-aminopyridine in EtOH (15 mL) was refluxed for 5h then allowed to cool and poured into ice cold water. The solid obtained was filtered off, dried and recrystallized from EtOH to afford compounds 18a-c.
5-(Benzo[d]thiazol-2-yl)-6-imino-1-(4-nitrophenyl)-4-oxo-1,4,5,6-tetrahydropyridine-3-carbonitrile(18a). Yield 75%; mp > 320°C; pale brown powder; IR (KBr) (ν/cm-1): 3350(NH), 2220(CN), 1700(C=O), 1610(C=C); 1H-NMR (DMSO-d6)δ(ppm): 4.28(s,1H,C-H), 6.40(d,2H,2Ar-CH,AB system), 7.20(d,2H,2Ar-CH,AB system), 7.50-8.00 (m,4H,Ar-H), 8.10(s,1H,CH) , 9.95(s,1H,NH); 13C-NMR (DMSO-d6)δ(ppm): 55.2,90.5,118.3,121.6,121.8,124.5,125.3,(2)123.9,(2)124.7,135.2,137.9,143,158.6, 152.8,156.4.168.7,196.5; MS(EI,70 eV): m/z (%) 389 (M+,80),384(50),284(50),237(40),21540),132(50). Anal. Calcd for C19H11N5O3S (389.39): C,58.61; H,2.85; N, 17.99%. Found: C, 58.42; H, 2.67; N, 17.80%.
5-(Benzo[d]thiazol-2-yl)-6-imino-4-oxo-1-(p-tolyl)-1,4,5,6-tetrahydropyridine-3-carbonitrile (18b). Yield 65%; mp>320°C, yellow sheets, IR(KBr) (ν/cm-1): 3350 (NH), 2220(CN), 1700(C=O), 1610(C=C); 1H-NMR(DMSO-d6) δ (ppm): 2.30 (s,3H,CH3), 3.60(s,1H,CH), 6.40(d,J=8.1Hz, 2H, Ar-H AB system), 7.20 (d,j=8.2 Hz, 2H, Ar-H AB system), 7.50-8.00 (m,4H,Ar-H), 8.70 (s,1H,=CH), 9.30 (s,1H,NH); MS(EI,70 eV): m/z(%) 358(M+,40), 316(100), 269(30), 242.10(70), 195(30). Anal. Calcd for C20H14N4OS (358.42): C, 67.02; H, 3.44; N, 15.63%. Found: C, 66.95; H, 3.25; N, 15.53%.
3-(Benzo[d]thiazol-2-yl)-2-imino-4-oxo-3,4-dihydro-2H-[1,2’-bipyridine]-5-carbonitrile (18c). Yield 65%; mp>330°C, yellow powder; IR (KBr) (ν/cm-1): 3350(NH), 2220(CN), 1700(C=O), 1610(C=C); 1H-NMR(DMSO-d6)δ(ppm): 4.22(s,1H,C-H), 6.43(d,2H,2Ar-CH,AB system), 7.50-8.00 (m,4H,Ar-H), 8.20(s, 1H,CH) , 9.84(s,1H,NH); MS (EI,70 eV): m/z (%)345 (M+,70), 327(30), 299(60), 283(50), 255(20), 227(60), 211(40), 155(35). Anal. Calcd for C18H11N5OS (345.38): C, 62.60; H, 3.21; N, 20.28%. Found: C, 62.50; H, 3.11; N, 20.15%.
Synthesis of 7-(benzo[d]thiazol-2-yl(cyano)methylene)-5-(dimethylamino)-1,4-oxathiepane-6-carbonitrile (19). A solution of compound 5 (2.96g, 0.01 mole) and 2-mercaptoethanol (0.78g, 0.01 mol) in boiling glacial acetic acid (15mL) was refluxed for 5h. The reaction was allowed to cool and poured into ice cold water. The solid obtained was filtered off, dried and recrystallized from EtOH to afford of compound 19; yield 65%; mp 210°C, orange powder; IR (KBr) (ν/cm-1): 2220, 2218 (2CN); 1H-NMR(DMSO-d6) δ (ppm): 2.27 (s,6H,2CH3), 2.76 (d, J=6.5 Hz, 1H, S-CH), 2.86 (d, J=6.8 Hz, 1H, C-CH), 3.68 (t, J=6.4 Hz, 2H, S-CH2), 3.70 (t, J=6.2 Hz, 2H, O-CH2), 7.55-8.23 (m, 4H, Ar-H), 13C-NMR (DMSO-d6) δ (ppm): 28.5, 43.3,67.1,68.4,73.4,116.8,118.8,121.6,124.5,125.3,136.2,153.5,160.5,175.3,MS(EI,70eV): m/z (%) 356(M+, 70),351(80),334(100),270(30),212(90),169(50). Anal. Calcd for C17H16N4OS2 (356.46): C, 57.28; H, 4.52; N, 15.72%. Found: C, 57.15; H, 4.535; N, 15.64%.
Antimicrobial screening
Standard sterilized filter paper disks (5mm diameter) impregnated with a solution of the tested compound in DMF (1mg/mL) was placed on an agar plate seeded with the appropriate test organism in triplicates. The utilized test organism was B. subtilis and B. thuringiensis as example of Gram-positive bacteria and E. coli and P. aeruginosa as example of Gram-negative bactaria. They were also evaluated for their in vitro antifungal potential against F. oxysporum and B. fabea fungal strains. Chloramphenicol, Cephalothin, Ampicillin and Cycloheximide were used as standard antibacterial and antifungal agents, respectively [22]. DMF alone was used as control at the same above mentioned concentration. The plates were incubated at 37°c for 24h for bacteria and 48 days for fungi. Compounds that showed significant growth inhibition zones (>14mm) using the two fold serial dilution technique, were further evaluated for their minimum inhibitory concentration (MICs) [21].
Minimum inhibitory concentration (MIC) and minimum bacterial concentration (MBC) measurements [21].
The microdilution susceptibility test in Muller-Hinton Broth (Oxoid) and Sabouraud Liquid Medium (Oxoid) was used for the determination of antibacterial and antifungal activity, respectively. Stock solutions of the tested compounds, chloramphenicol, cephalothin and cycloheximide were prepared in DMF at concentration of 1000 μg/mL. Each stock solution was diluted with standard method broth (Difco) to prepare serial two fold dilutions in the range of 500-3125 μg/mL 10 mL of broth containing about 106 CFU/Ml of test bacteria was added to each well of 96-well microtiter plate. The sealed microplates were incubated at 37°C for 24h for antibacterial activity and at 37°C for 48h for antifungal activity in a humid chamber. At the end of incubation period, the minimal inhibitory concentration (MICS) values were recorded at the lowest concentration of the substance that had no visible turbidity. Control experiments with DMF and uninoculated media were run parallel to the test compounds under the same conditions, as shown in tables 1 and 2.
| Table 2: The minimum bacterial concentration (MBC, μg/mL) of some new synthesized compounds. | ||||||
| Compound No. | MBCa in μg/Ml | |||||
| Bacteria | Fungi | |||||
| Gram-positive bacteria | Gram-negative bacteria | |||||
| B. subtilis | B. thuringiensis | E. coli | P. ueruginosa | F. oxysporum | B. fabea | |
| 3 | 200 | b | 200 | b | b | b |
| 4 | 200 | b | 200 | b | b | b |
| 5 | 200 | b | 200 | b | b | b |
| 6 | 100 | b | 100 | b | b | b |
| 7 | 50 | b | 200 | b | b | b |
| 8 | 200 | b | 200 | b | b | b |
| 9 | 200 | b | 200 | b | b | b |
| 10 | 50 | b | 200 | b | b | b |
| 11 | 200 | b | 100 | b | b | b |
| 12 | 50 | b | 50 | b | b | b |
| 13 | 200 | b | 100 | b | b | b |
| 14 | 200 | b | 200 | b | b | b |
| 15 | 200 | b | 200 | b | b | b |
| 16 | 200 | b | 100 | b | b | b |
| 17a | 200 | b | 200 | b | b | b |
| 17b | 200 | b | 200 | b | b | b |
| 18a | 100 | b | 200 | b | b | b |
| 18b | 100 | b | 200 | b | b | b |
| 18c | 100 | b | 50 | b | b | b |
| Ampicillin | 50 | b | 25 | b | b | b |
| aMBC: Minimum bacterial concentration (the lowest concentration at which no bacterial growth was observed). bNT: Not tested |
||||||
Acknowledgements
The authors owe to Department of Pharmacology, Faculty of Pharmacy, Mansoura University, Egypt for performing the antimicrobial evaluation.
References
- Papesch V, Schroeder E. Synthesis of 1 Mono and 1,3 Di Substituted 6 Amino Uracils. Diuretic Activity 1. J Org Chem. 1951; 16: 1879-1890. Ref.: https://goo.gl/PejvGJ
- Muller T, Augustin M, and Werchan HGZ. Zur Nitrosierung N′-aryl-substituierter N-Cyanacetyl-harnstoffe; Ringschluß und -transformation von Uracil-Derivaten. Zeitschrift für Chemie, 1989; 29: 281-283. Ref.: https://goo.gl/uqr2KF
- Isobe Y, Tobe M, Inoue Y, Isobe M, Tsuchiya M, et al. Structure and activity relationships of novel uracil derivatives as topical anti-inflammatory agents. Bioorg Med Chem 2003; 11: 4933-4940. Ref.: https://goo.gl/knHHn4
- Kappe T, Stelzel HP, Ziegler E. Synthese von Pyridonen aus Enaminen und Cyanessigsäuren. Montsh Chem. 1983; 114: 953-963. Ref.: https://goo.gl/yaY13h
- Stetinova J, Kada R, Lesko J, Zalibera L, Ilavský D et al. Synthesis and properties of substituted 1-(2-benzothiazolyl)-2-pyridones. Collect Czech Chem Commun. 1995; 60: 999-1008. Ref.: https://goo.gl/gwkpac
- Al-Lohedan H, Bunton CA. Micellar effects upon the ElcB mechanism of ester hydrolysis. J Org Chem. 1981; 46: 3929-3930. Ref.: https://goo.gl/eMhqLH
- Refat HM, Fadda AA. Synthesis and Cytotoxicity Activity of Some Novel Hydrazide, Pyrazole, Isoxazole, Pyrimidine and Fused Pyran-2-one Derivatives. Heterocycles. 2015; 91: 1212-1226. Ref.: https://goo.gl/ZZ4Ro3
- Fadda AA, El-Mekawy RE, El-Shafei AI. Synthesis, antiviral, cytotoxicity and antitumor evaluations of A4 type of porphyrin derivative. J Porphirins Phthalocyanins. 2015; 19: 753-768. Ref.: https://goo.gl/HkbGMb
- Fadda AA, Amer FA, Zaki MEA. Revised synthesis of some new 2-heterocyclic benzothiazolyl derivatives of biological interest. Phosphorus, Sulfur Silicon Relat. Elem. 1999; 155: 59-66. Ref.: https://goo.gl/bGCDAu
- Fadda AA, Abdelrazek FM, Mohamed kh S, Mostafa HM, Etman HA, et al. Synthetic applications of benzothiazole containing cyanoacetyl group. Eur J Chem. 2010; 1: 90-95. Ref.: https://goo.gl/Y6nM2m
- Mohamed Kh S, Abdulaziz NM, Fadda AA. Synthesis of New Heterocyclic rings Containing Benzothiazole Moiety. J Heterocycl Chem. 2013; 50: 650-653. Ref.: https://goo.gl/xWdAZW
- Mohamed Kh S, Abdulaziz NM, Fadda AA. Synthesis of Some New Pyridine and Pyrimidine Derivatives Containing Benzothiazole Moiety. J Heterocycl Chem. 2013; 50: 645-649. Ref.: https://goo.gl/fi591B
- Fadda AA, Hammouda M, Afsah EM. Nitriles in Organic Synthesis: Synthesis of New Benzothiazole Derivatives of Biological Interest. Phosphorus, Sulfur Silicon Relat Elem. 2010; 185: 433-446. Ref.: https://goo.gl/cNjCVY
- Fadda A A, Abdel-latif E, Bondock S. Synthesis of Some New Pyrimidine and Pyrimido [4,5-d] pyrimidine Derivatives. Synth Commun. 2008; 38: 4352-4368. Ref.: https://goo.gl/AZ6u6d
- Fadda AA, Zaki MEA, Mohamed Kh S, Amer FA. Nitriles in Organic Synthesis: Synthesis of Some New 2-Heterocyclic Benzothiazol Derivatives. Phosphorus Sulfur Silicon Relat Elem. 2007; 182: 1845-1856. Ref.: https://goo.gl/Ww6CCC
- Zaki MEA, Fadda AA, Mohamed Kh S. Nitriles in Organic Synthesis: A Convenient Route to Some Heterocycles Incorporating a Benzothiazole Moiety. Phosphorus Sulfur Silicon Relat Elem. 2006; 181: 1815-1823. Ref.: https://goo.gl/kwhTYF
- Zaki MEA, Fadda AA, Samir k, Amer FA. Nitriles in Organic Synthesis: Synthesis of Pyrido[2,1-b]Benzothiazole Derivatives and Polyfunctionally Substituted Pyridines. Chem Heterocycl Compd. 2003; 39: 1242-1248. Ref.: https://goo.gl/5YxWk3
- Abdel-latif E, Mustafa HM, Etman HA, Fadda AA. Synthesis of new Purin, Pteridine and other Pyrimidine Derivatives. Russ J Org Chem. 2007; 43: 443-448. Ref.: https://goo.gl/4phkvA
- Fadda AA, Refat HM, Mohamed, Kh S. Synthesis and Antimicrobial Evaluation of Some New Dihydropyridine, Pyrazole, Chromene, Pyrrole, Thiazole and Thiophene Derivatives. Heterocycles. 2014; 89: 2318-2333. Ref.: https://goo.gl/4916UZ
- Bondock S, Khalifa W, Fadda AA. Synthesis and Antimicrobial Evaluation of Some New Thiazole, Thiazolidinone and Thiazoline Derivatives Starting from 1-Chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur J Med Chem. 2007; 42: 948-954. Ref.: https://goo.gl/M9kgFU
- Abo-Melha H, Fadda AA. Synthesis, spectral characterization and in vitro antimicrobial activity of some new azopyridine derivatives. Spectrochim Acta Part A. 2012; 89: 123-128. Ref.: https://goo.gl/iGYC7h
- Fadda AA, Zaki M, Mohamed Kh S, Samir K, Etman HA. Chemistry of 2-Cyanomethyl-benzothiazole.Phosphorus, Sulfur Silicon Relat Elem. 2008; 183: 1801-1842. Ref.: https://goo.gl/tk6yQ6