More Information
Submitted: July 25, 2022 | Approved: August 30, 2022 | Published: September 02, 2022
How to cite this article: Javidfar F, Fadaeian M. Fabrication of novel Co3O4@GO/La2O3 nanocomposites as efficient, innovative and recyclable nanocatalysts for the synthesis of quinazolinone derivatives under solvent-free conditions. Ann Adv Chem. 2022; 6: 043-047.
DOI: 10.29328/journal.aac.1001030
Copyright License: © 2022 Javidfar F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Quinazolinone derivatives; Heterogeneous; Lanthanum; Magnetic nanocomposite
Fabrication of novel Co3O4@GO/La2O3 nanocomposites as efficient, innovative and recyclable nanocatalysts for the synthesis of quinazolinone derivatives under solvent-free conditions
Fereshteh Javidfar and Manoochehr Fadaeian*
Department of Chemistry, Qom Branch, Islamic Azad University, Qom, Iran
*Address for Correspondence: Dr. Manoochehr Fadaeian, Department of Chemistry, Qom Branch, Islamic, Azad University, Qom, Iran, Email: [email protected]; [email protected]
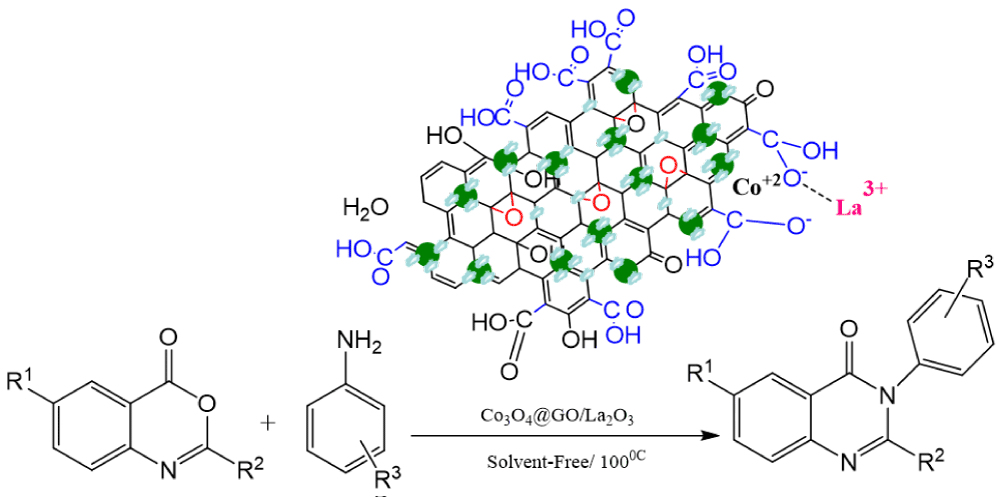
For the first time, this research has developed an efficient and novel approach to high to excellent yields for synthesizing Quinazolinone derivatives. Also, the synthesis of Quinazolinone derivatives has been carried out in the presence of Co3O4@GO/La2O3 nanocomposite as a novel heterogeneous catalyst and a green under solvent-free conditions and in a short time and excellent yields for the first time. Various structural and morphological characteristics of the nanocatalyst were employed for the catalyst characterization, such as FT-IR, XRD, FE-SEM, EDX and VSM analyses. All characterization data were checked with each other so that the structure of the nanocatalyst was exactly characterized. The reactions were carried out in the presence of a low amount of nanocatalyst at 100 °C under solvent-free conditions for a short period of time. The proposed nanocomposite exhibits excellent catalytic activity. One of the most important advantages of this method is easy magnetic nanocatalyst separation, green condition, excellent recoverability and easy workup.
In recent years, one of the main challenges facing scientists is to make the materials they utilize as environmentally friendly as possible. Therefore, the idea of green chemistry is raised [1].
Graphene, the thinnest and strongest material in the world, was discovered in 2004 [2]. Graphene has a two-dimensional (2D) structure of a honeycomb lattice layer of carbon atoms due to unique properties such as adjustable surface properties, high thermal stability, and excellent electronic conductivity. Graphene is an excellent candidate for catalysts. One of the most important derivatives of graphene is graphene oxide (GO), which contains hydroxyl and epoxide groups and carboxyl groups that organic molecules can modify and metal oxide nanoparticles with magnetic properties [2,3]. Graphene oxide (GO) was attention as an excellent carrier for transition metal ions with high conductivity, high mechanical and wide specific surface area [4].
Recently, cobalt oxide nanoparticles (Co3O4) are an important p-type magnetic semiconductor due to their properties such as low cost, good electrical conductivity, environmental compatibility, simple preparation, and high chemical and physical stability. It has been increasingly considered a catalyst in various reactions [5].
Lanthanides were discovered in 1794 [6]. Lanthanum oxide (La2O3), due to its properties such as unique electronic and basic structure, excellent paramagnetic sensitivity, saturated magnetization, and the largest band gap, can be an excellent candidate for improving catalytic activity [3,6,7].
Recently, Quinazolinones have attracted the attention of chemists due to their unique properties, such as antifungal, antimalarial, antihypertensive, anticonvulsant and antiparkinson [8,9].
In this work, we report a simple and efficient method for synthesizing quinazolinone derivatives in the presence of Co3O4/GO/La2O3 nanocomposite as a novel nanocatalyst under solvent-free conditions at 100 °C.
Sigma Aldrich and Merck prepared all solvents and reagents used in this work with excellent purity. Melting point (m.p) was operated by Electro-Thermal 9200 and FT-IR analysis was obtained by PerkinElmer 1600 FTIR spectrometer. Bruker confirmed 1 H and 13 C Nuclear Magnetic Resonance (NMR) spectroscopy at 300 and 75 MHz, respectively (in DMSO, internal reference: TMS). Philips-X’pertpro, an X-ray diffractometer utilizing Ni-filtered Cu Kα radiation, was used for operating X-ray Diffraction (XRD) analysis at a scanning speed of 2 min1 from 10 to 100 (2Ɵ). Magnetic properties were characterized by a Vibrating Sample Magnetometer (VSM, Taban laboratory, Tehran, Iran) at room temperature. In addition, the morphology and size of samples were investigated by the Scanning Electron Microscope (SEM) and Energy-Dispersive Spectroscopy (EDS, EDX).
Preparation of Co3O4 nanoparticles
According to previous literature, Co3O4 NPs were synthesized was reported to be successful [10]. At the first step, in a round bottom flask, about 8.60 g of cobalt (II) nitrate was dissolved in 100 ml of Ethanol for about half a min. After this step, about 2.14 g of Ethane dioic acid was slowly added, and the reaction mixture was stirred for 120 min at 45 °C. The precipitate obtained containing cobalt oxalate (II) (CoC2O4) powder was collected by centrifuge followed by calcined at 400 °C for 120 min to generate Co3O4 nanoparticles.
Preparation of Co3O4@GO
Co3O4@GO nanocomposite was fabricated by dispersing about 0.5 g of Co3O4 in 25 mL deionized water that was added 0.1 g wise into GO (50 mL, aq.) by the Hummer method [11]. After that, the reaction mixture was stirred for 60 min at 60 °C. In the final stage, the nanocomposite was separated by an external magnet, washed three times with deionized water and dried [3].
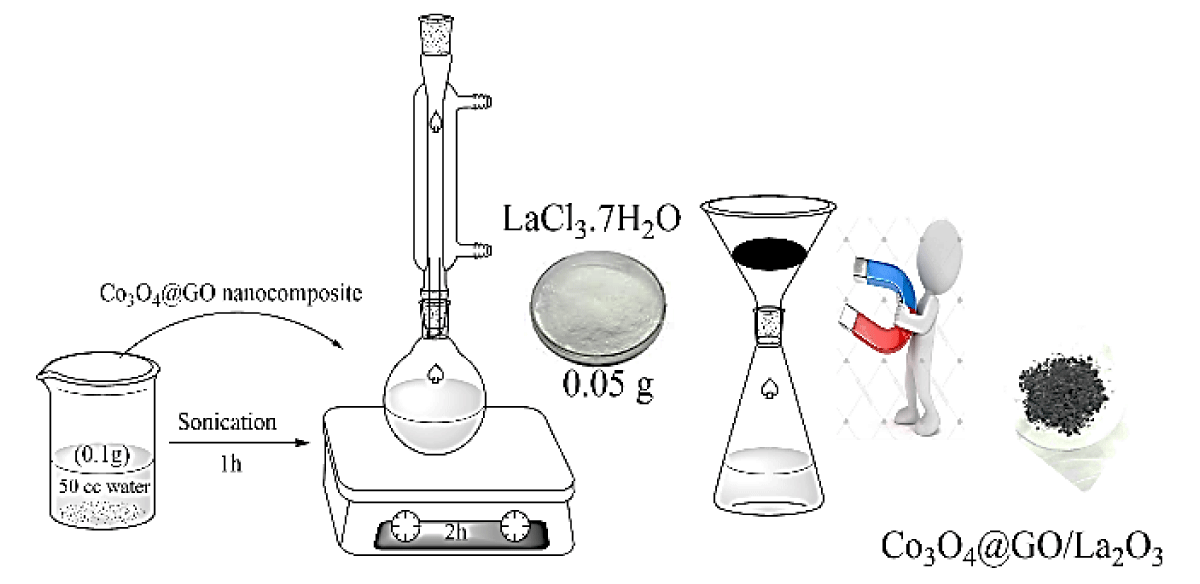
Procedure for the preparation of Co3O4@GO/La2O3
According to previous literature, Co3O4@GO/La2O3 nanocomposites were synthesized. Nanocomposites were synthesized [3,7] -approximately 0.1 g Co3O4@GO nanocomposites in 50 mL deionized water and sonicated for 60 min. About 0.05 g of LaCl3.7H2O was added and the reaction mixture was stirred under reflux conditions for 120 min. Fabricated nanocomposites were collected through an external magnet and then washed thrice with distilled water Scheme 1.
Scheme 1: Preparation of Co3O4@GO/La2O3.
General procedure for the synthesis of quinazolinone
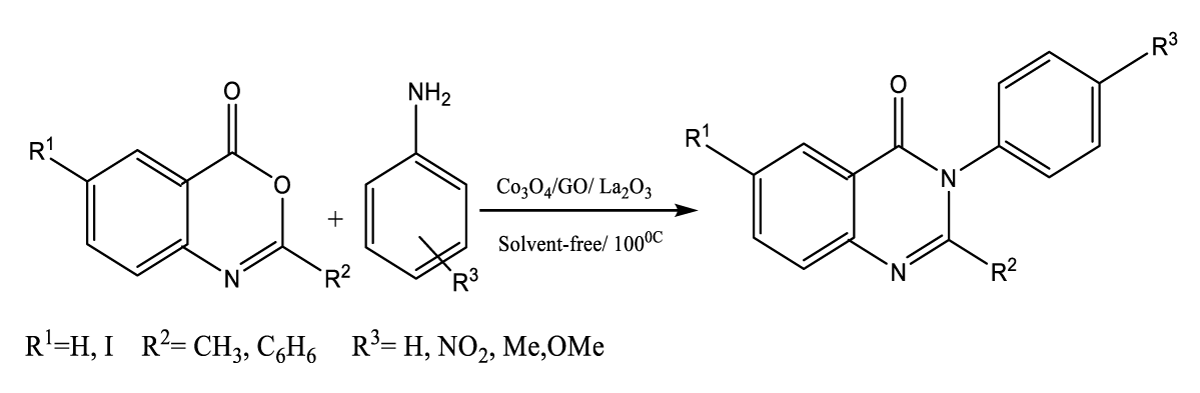
Typically, 1,3-benzoxazine-4-one derivatives (0.001 mol), aniline derivatives (0.001mol) and Co3O4@GO/La2O3 (0.05 g) were added to the tube and prepared in an oil bath at 100°C. The reaction mixture was stirred using a magnetic stirrer continuously. The thin-layer chromatography technique checked the reaction mixture and then the catalyst was separated by an external magnet. Finally, the product was recrystallized with hot ethanol to obtain a pure product (Figure 1). All products were investigated by Melting Point (m. p), 1H NMR, 13C NMR and FTIR. The spectral data of all are provided.
Figure 1: Synthesis of benzoxazine-4-one, aniline, in the presence of Co3O4/GO/ La2O3 under solvent-free conditions and 100.
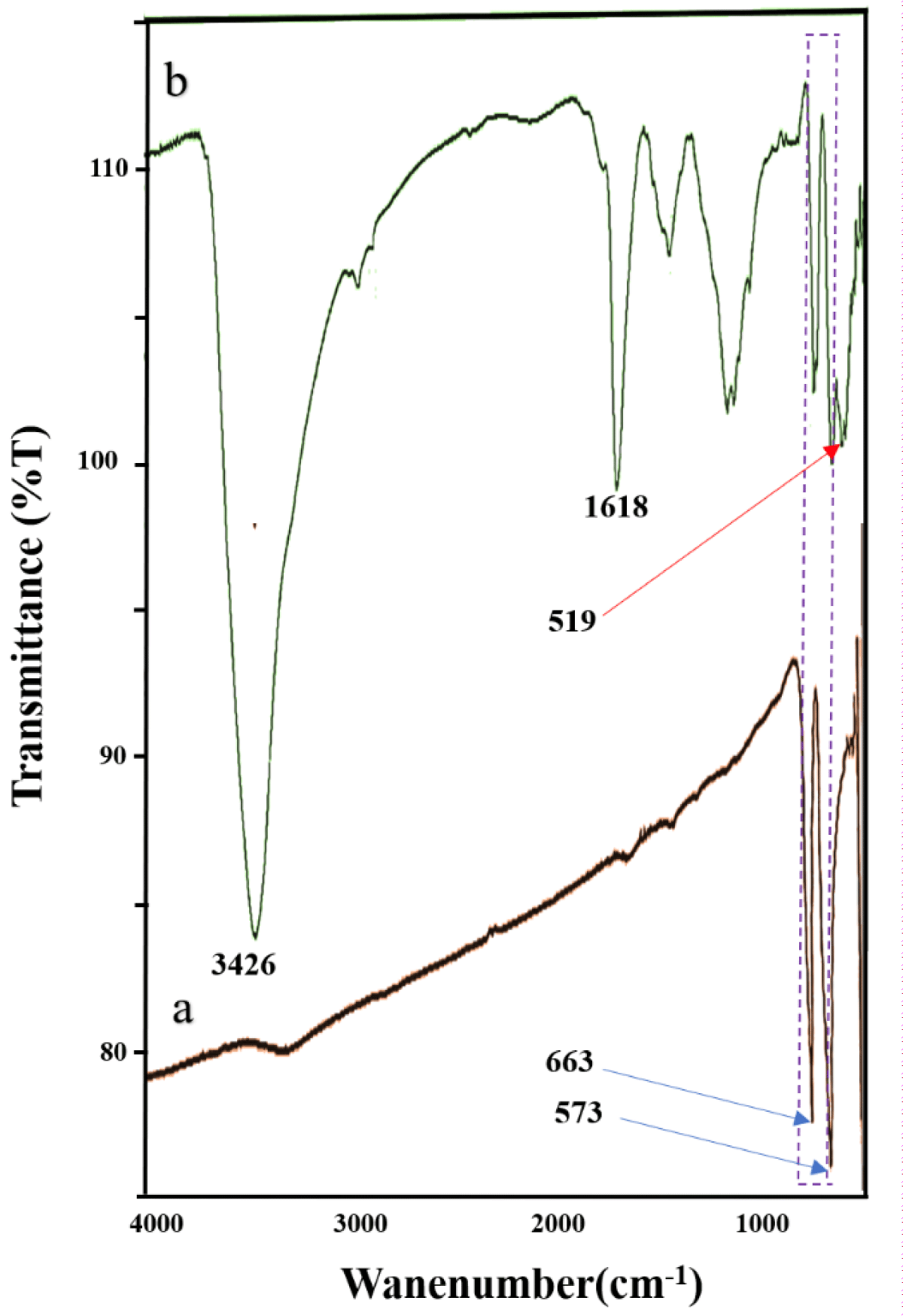
The FT-IR spectra are utilized to analyze the prepared nanocrystals. FTIR spectra of the Co3O4 NPs and Co3O4@GO/La2O3 are shown in Figure 2. Two strong characteristic absorptions at 573 and 663 cm-1 belong to the Co-O (Figure 2a,b). The absorption at 1618 cm-1 (aromatic C=C) can belong to skeletal vibrations of graphitic domains. Also, the peaks around 3426 cm-1 ascribe to the OH group bands in GO (Figure 2b) [11]. The characteristic absorptions showed at 519 cm-1 belong to the formation of La2O3, in which the range between 400 and 660 cm-1 corresponds to the literature (Figure 2b) [12].
Figure 2: FT-IR spectra of Co3O4 NPs (a), Co3O4/GO/ La2O3 (b).
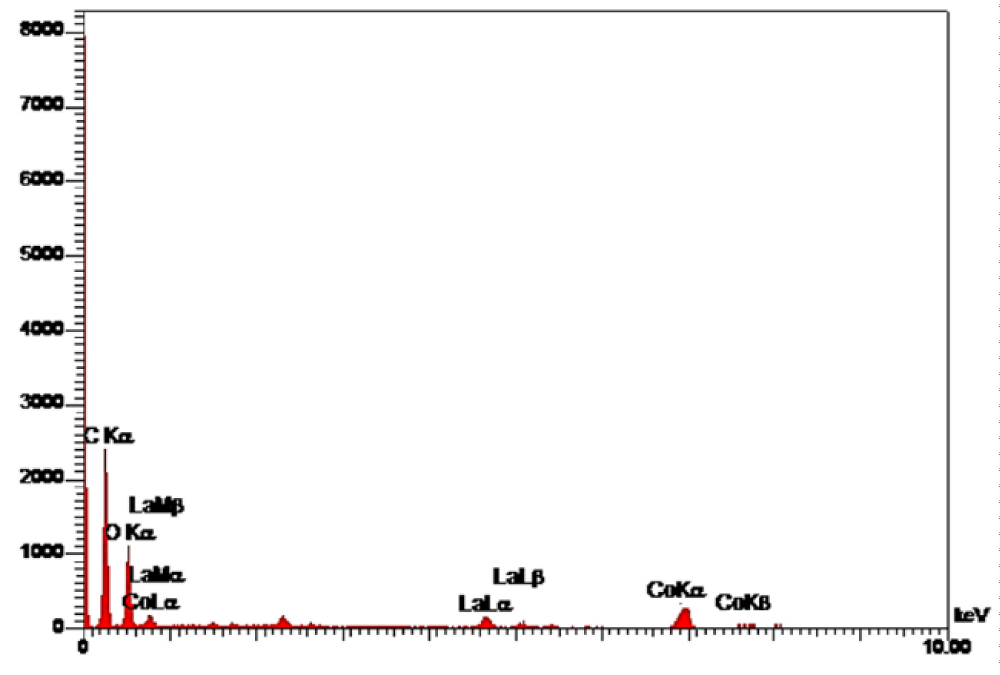
In the EDS spectrum observed in Figure 3, the presence of elements C, O, Co and La is determined according to the energy, demonstrating the product purity.
Figure 3: EDS spectrum of Co3O4/GO/ La2O3.
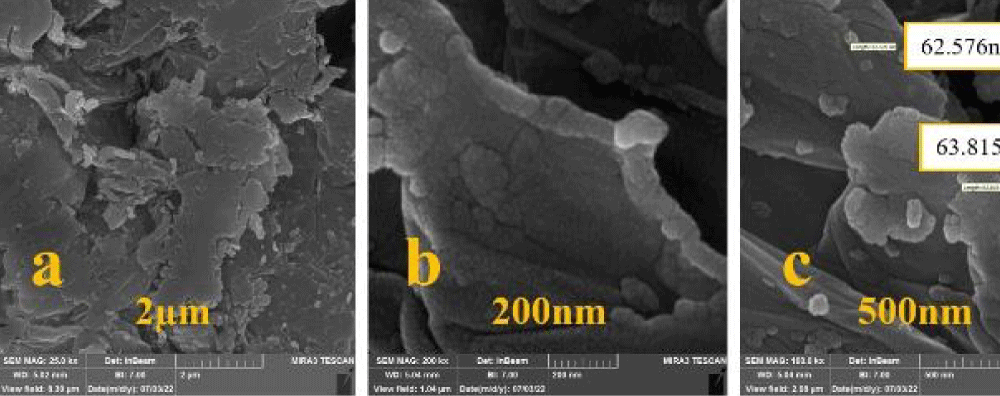
FESEM was utilized to measure the morphology, particle size and shape. The catalyst particle size was measured at about 62.5 nm. A uniform spherical shape was observed for Co3O4@GO/La2O3 nanocomposites (Figure 4).
Figure 4: FESEM spectrum of Co3O4/GO/ La2O3 (a-c).
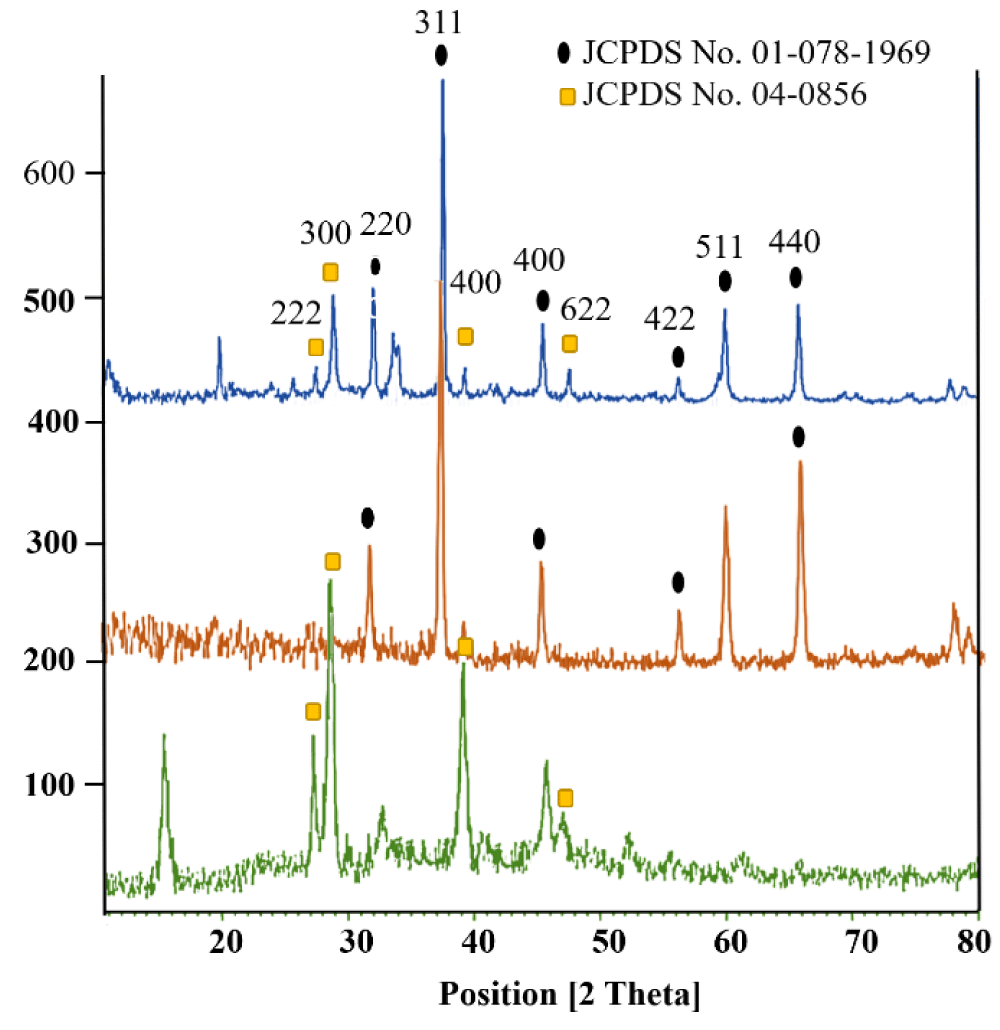
The data relating to the XRD analysis is shown for samples La2O3, Co3O4 nanoparticles and Co3O4@GO/La2O3 nanocomposite in Figure 5(a-c). Characteristic peaks for Co3O4 are shown in the region at 2θ of 31.47°, 37.04°, 45.01°, 55.86°, 59.57° and 65.44°, which correspond to (220), (311), (400), (422), (511) and (440) respectively. The network parameters were in excellent agreement with JCPDS card number 01-078-1969 [13]. The broad diffraction peaks of GO indicate around 2θ= 10.26 ° and it is in good match with the previous reports [3,14]. Also, La2O3 NPs had peaks that are explained at 27.2912°, 28.3643°, 39.6213° and 48.1237° are related to La2O3, which correspond to (222), (300), (400) and (622) respectively (La2O3; JCPDS card no.04-0856) [15] Characteristic peaks for Co3O4 and La2O3 illustrate that the structure of both of them has not changed during the combination process.
Figure 5: XRD patterns of a) La2O3 b) Co3O4 and c) Co3O4@GO/La2O3.
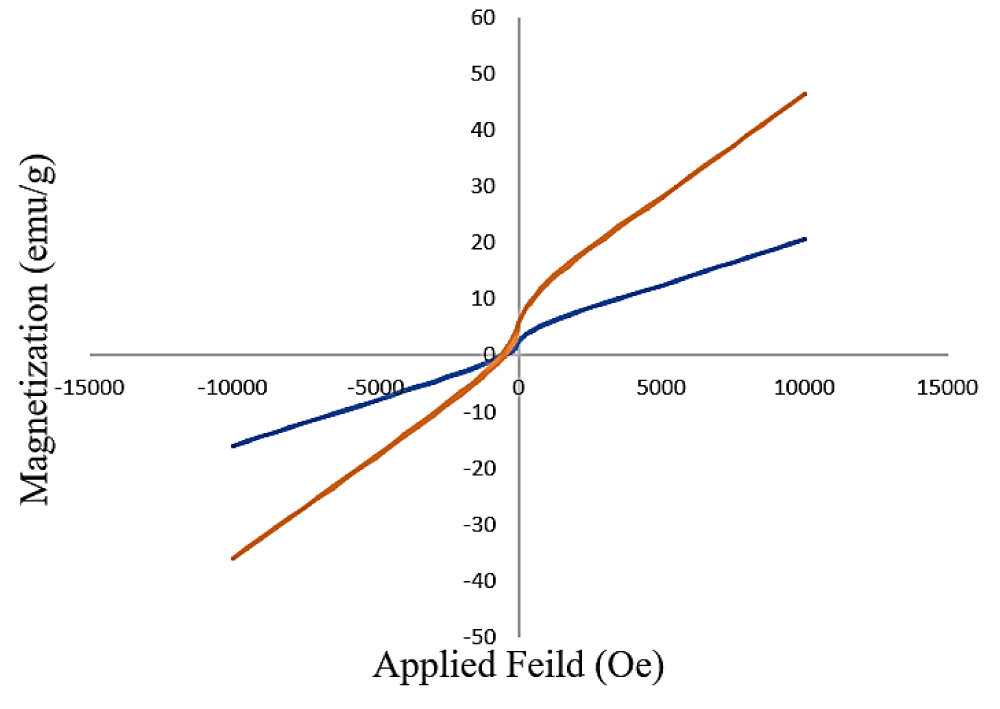
The magnetic behaviors of Co3O4 NPs and Co3O4@Cs/La2O3 were shown by a vibrating sample magnetometer (VSM) at room temperature (Figure 6). There is no hysteresis, coercivity, and remanence in bare Co3O4 NPs and Co3O4@Cs/La2O3 samples at room temperature that show their typical superparamagnetic behaviors. Co3O4 NPs and Co3O4@Cs/La2O3 have superparamagnetic behavior, facilitating magnetic separation. The saturation magnetization value (Ms) Co3O4 NPs and Co3O4@Cs/La2O3 nanocomposites were 46.5 and 25.8 emu/g, respectively. Howbeit, the addition of the GO layer and La2O3 nanoparticles on the Co3O4 surface Caused decreased magnetic behaviors. Co3O4 NPs and Co3O4@Cs/La2O3 nanocomposite saturation magnetization was enough for a rapid magnetic separation by an external magnet.
Figure 6: VSM (a) Co3O4, (b) Co3O4@GO/La2O3 nanocomposites.
As shown, Table 1 summarizes the results from the reaction of 1,3-(4H)-benzoxazine-4-one derivatives and aniline derivatives in the presence of Co3O4@GO/La2O3. A wide range of 1,3-(4H)-benzoxazine-4-one derivatives were successfully converted into Quinazolinone Derivatives with great yields within a short reaction time (Table 1, entries 1–10).
| Table 1: Synthesis of Quinazolinone Derivatives. | ||||||
| Entry | R1 | R2 | R3 | Time(min) | Yields% | Melting point |
| 1 | H | CH3 | NO2 | 15 | 87 | 158 - 159 |
| 2 | H | CH3 | CH3 | 18 | 83 | 115 - 116 |
| 3 | H | C6H5 | NO2 | 20 | 90 | 126 - 127 |
| 4 | H | C6H5 | CH3 | 20 | 90 | 209 - 210 |
| 5 | I | C6H5 | CH3 | 15 | 93 | 183 - 184 |
| 6 | H | C6H5 | OCH3 | 17 | 87 | 222 - 223 |
| 7 | I | C6H5 | OCH3 | 20 | 89 | 165 - 166 |
| 8 | H | C6H5 | H | 15 | 70 | 172 - 173 |
| 9 | I | C6H5 | NO2 | 20 | 80 | 150 - 151 |
| 10 | I | C6H5 | H | 20 | 74 | 138 - 139 |
| Reaction conditions: 1,3-benzoxazine-4-one derivatives (0.001 mol), aniline derivatives (0.001mol), Co3O4@GO/La2O3, at 100 °C under Solvent-Free conditions. | ||||||
In Table 2, a vast comparison of catalytic activity Co3O4@GO/La2O3 was made with other conditions of reactions utilized in the preparation of quinazolinone (Table 2, entry 12-20). In conclusion, the catalyst has excellent results and offers a very gentle and green option.
Table 2: Comparing the efficiency of Co3O4@GO/La2O3 with other reactions reported in the literature for the synthesis of quinazolinone.
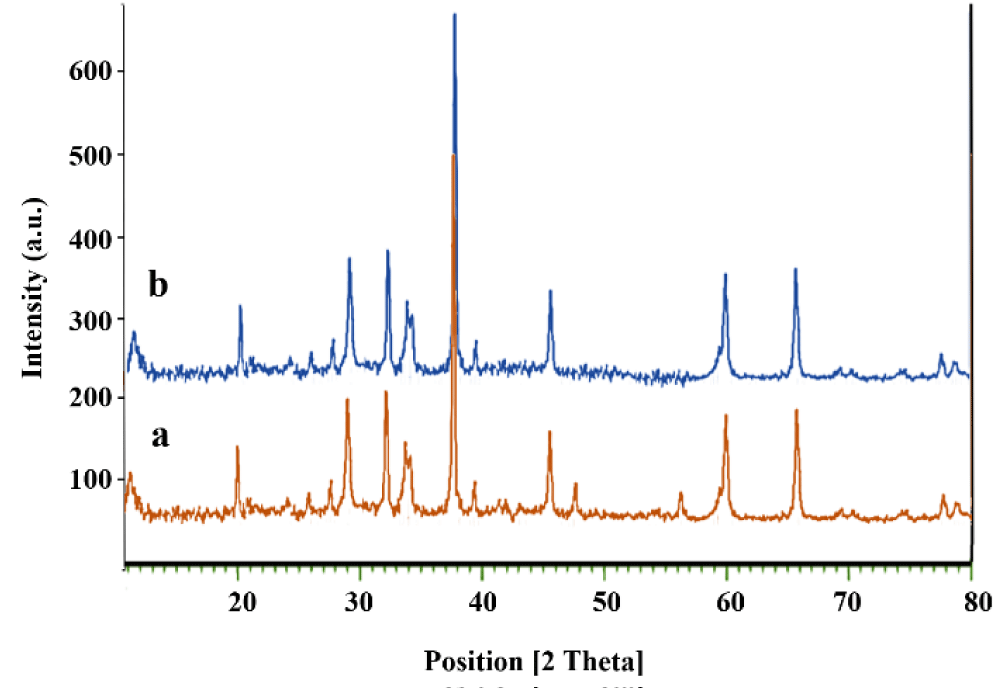
XRD analysis of the Co3O4@GO/La2O3 nanocatalysts after five reuse cycles is indicated in Figure 7b. Characteristic peaks for Co3O4@GO/La2O3 are conserved, displaying that the nanocatalysts have been stable and pure.
Figure 7: Comparison of XRD of the prepared Co3O4@GO/La2O3 (a) before and (b) after 5 runs.
Briefly, a highly efficient, powerful and green protocol was developed to synthesize 1,3-(4H)-benzoxazine-4-one derivatives and aniline derivatives under solvent-free conditions at 100 °C using novel Co3O4@GO/La2O3 nano-catalyst. The magnetically recoverable nanocatalyst was characterized by FT-IR, FE-SEM, XRD, EDX and VSM analyses. This nanocatalyst has various advantages, such as being eco-friendly, having mild reaction conditions, low cost, high efficiency and being easily separated magnetically.
Supplementary materials: The 1H-NMR and 13C-NMR spectrums of compounds are available online.
Thanking: The authors are commendable for the financial support of the Research Council of the Islamic Azad University, Qom, Iran.
- Al-Toum R, Bdour S, Ayyash H. Adenovirus Infections in Jordanian Hospitalized Paediatric Patients: Prevalence and Clinical Features. J Med J. 2009; 43(3):171–179.
- Ighilahriz-Boubchir K, Boutemeur-Kheddis B, Rabia C, Makhloufi-Chebli M, Hamdi M, Silva AMS. Recyclable Keggin Heteropolyacids as an Environmentally Benign Catalyst for the Synthesis of New 2-Benzoylamino-N-phenyl-benzamide Derivatives under Microwave Irradiations at Solvent-Free Conditions and the Evaluation of Biological Activity. Molecules. 2017 Dec 21;23(1):8. doi: 10.3390/molecules23010008. PMID: 29267237; PMCID: PMC5943967.
- Shi P, Dai X, Zheng H, Li D, Yao W, Hu Ch. Synergistic catalysis of Co3O4 and graphene oxide on Co3O4/GO catalysts for degradation of Orange II in water by advanced oxidation technology based on sulfate radicals. Chem. Eng. J. 2014; 240: 264–270.
- Javidfar F, Fadaeian M, Safaie Ghomi J. Synthesis of Fe3O4@GO Nanocomposites Modified with La2O3 Nanoparticles as an Efficient Catalyst for Selective Oxidation of Aromatic Alcohols to Aldehydes. Polycycl, Aromat. Compd. 2021.
- Can K, Fang L, Hui S, Zhou Zh, Benjie Z, Wei Li. Co3O4/GO catalyst as efficient heterogeneous catalyst for degradation of wastewater containing polyacrylamide (PAM). Water Cycle. 2021.
- Islami M, Zarrabi A, Tada S, Kawamoto M, Isoshima T, Ito Y. Controlled quercetin release from high-capacity-loading hyperbranched polyglycerol-functionalized graphene oxide. Int J Nanomedicine. 2018 Oct 5;13:6059-6071. doi: 10.2147/IJN.S178374. PMID: 30323593; PMCID: PMC6179725.
- Gadolin J. Examination of a black, dense mineral from the Ytterby Quarry in Roslagen. Proceedings of the Royal Academy (Stockholm, new series) (in the original Swedish: Undersökning af en svart tung Stenart ifrån Ytterby Stenbrott i Roslagen. K Vetenskaps-akad nya handl). 1794; 15: 137-155.
- Javidfar F, Fadaeian M, Ghomi JS. La(OH)3 nanoparticles immobilized on Fe3O4@chitosan composites as novel magnetic nanocatalysts for sonochemical oxidation of benzyl alcohol to benzaldehyde. RSC Adv. 2021 Nov 8;11(57):35988-35993. doi: 10.1039/d1ra05848g. PMID: 35492745; PMCID: PMC9043185.
- Khosropour AR, Mohammadpoor-Baltork I, Ghorbankhani H. Bi (TFA)3–[nbp]FeCl4: a new, efficient and reusable promoter system for the synthesis of 4(3H)-quinazolinone derivatives.Tetrahedron. Lett. 2006; 47(21): 3561–3564.
- Narasimhulu M, Mahesh KC, Reddy TS, Rajesh K, Venkateswarlu Y. Lanthanum (III) nitrate hexahydrate or p-toluenesulfonic acid catalyzed one-pot synthesis of 4(3H)-quinazolinones under solvent-free conditions. Tetrahedron. Lett. 2006; 47(26): 4381–4383.
- Lin CC, Guo Y, Vela J. Microstructure Effects on the Water Oxidation Activity of Co3O4/Porous Silica Nanocomposites. ACS Catal. 2015; 5(2): 51037–1044.
- Zhou X, Shi J, Qin PH, Fan YF, Min JC, Yao YL, WF. Synthesis of Co3O4/graphene composite catalysts through CTAB-assisted method for orange II degradation by activation of peroxymonosulfate. J. Mater. Sci. Mater. Electron. 2016; 27: 1020–1030.
- Abdoli M, Nami N, Hossaini Z. One-Pot Synthesis of Spiro-Acridine/Indoline and Indoline Derivatives Using (MWCNTs) - COOH/La2O3 Hybrid as an Effective Catalyst. J. Heterocycl. Chem. 2020; 58(2): 523–33.
- Aftab U, Tahira A, Gradone A, Morandi V, Abro MI, Baloch MM, Ibupoto ZH. Int. J. Hydrog. Energy. 2021; 46(13): 9110–9122.
- Vinodha G, Shima PD, Cindrella L. Mesoporous Magnetite Nanoparticle-Decorated Graphene Oxide Nanosheets for Efficient Electrochemical Detection of Hydrazine. J. Mater. Sci. 2019; 54: 4073–88.
- Tang B, Ge J, Wu C, Zhuo L, Niu J, Chen Z, Shi Z, Dong Y. Sol–Solvothermal Synthesis and Microwave Evolution of La (OH)3 Nanorods to La2O3 nanorods. Nanotechnology. 2004; 15(9):1273–6.
- Dai X, Virgil S. Synthesis of 2-heterosubstituted quinazolinone atropisomeric phosphine ligands by direct lithiation of a 2-unsubstituted quinazolinone system. Tetrahedron: Asymmetry. 1999; 10(1): 25–29.
- Abbas SY, El-Bayouki KAM, Basyouni WM, Mostafa EA. New series of 4(3H)-quinazolinone derivatives: syntheses and evaluation of antitumor and antiviral activities. Med Chem Res. 2017; 27: 571–582.
- Norouzi FH, Foroughifar N, Khajeh-Amiri A, Pasdar H. A novel superparamagnetic powerful guanidine-functionalized γ-Fe2O3 based sulfonic acid recyclable and efficient heterogeneous catalyst for microwave-assisted rapid synthesis of quinazolin-4(3H)-one derivatives in Green media. RSC Adv. 2021 Sep 7;11(48):29948-29959. doi: 10.1039/d1ra05560g. PMID: 35480261; PMCID: PMC9040894.